Report of Exosomes as Cell-Free Vaccines
Human Vaccines & Immunotherapeutics. 2024 Dec 31; 20 (1): 2345940.
Authors: Dai Z, Cai R, Zeng H, Zhu H, Dou Y, Sun S
INTRODUCTION
Traditional vaccines have limits against some persistent infections and pathogens. The development of novel vaccine technologies is particularly critical for the future. Exosomes play an important role in physiological and pathological processes. Exosomes present many advantages, such as the inherent capacity being biocompatible, and non-toxic, which make them a more desirable candidate for vaccines. However, research on exosomes is in its infancy and the barriers of low yield, low purity, and weak targeting of exosomes limit their applications in vaccines.
There are two ways to load proteins onto exosomes. One way is feeding antigens to dendritic cells (DCs) and then collecting the supernatants to separate exosomes for indirect loading of peptides, which ensures that antigen peptide binds to MHC molecules. MHC molecules are crucial for the interaction between DCs and T cells. However, the limitation of this strategy is that the type of antigen must be recognized by the DCs. The other way is that any soluble antigen can be attached to the exosome and can be used to modify exosomes with additional functions, such as adding adhesion molecules to exosomes, which can be targeted to certain sites.
Advantages and Disadvantages of Vaccine Types
| Vaccine types | Advantages | Disadvantages |
| Inactivated vaccine | Mature technology; Relatively simple to construct; Inactive ingredient | Booster immunization is needed |
| Adenoviral vector vaccine | Mass production; Multiple epitopes can be induced simultaneously; Strong immunogenicity | Relatively complex to construct; Pre-existing anti-adenovirus immune responses with potential adverse events |
| DNA vaccine | Vectors can be constructed to encode different antigens in a single vaccine; Mass production, low cost, high stability of production and storage; No active ingredients, no risk of vaccine-triggering disease | Weak immune response; The delivery agent is required to reach the nucleus |
| RNA vaccine | Rapid development; Novel mRNA sequences can be quickly assembled into existing vaccine formulations; No risk of integration with host cell genes; No active ingredients, and no risk of vaccine-triggering disease | High cost; Lipid nanoparticles must be stored at ultra-low temperature; Prone to severe and rare allergic reactions |
| Recombinant protein vaccine | Mature technology; Mass production; No active ingredients; Relatively stable | Adjuvants required; Elicit weak immune response; Only protein fragments are expressed, not all proteins; Screening for the best combination of antigens takes time |
| EV-based vaccine | Self-presenting antigen; Crossing the blood-brain barrier; High absorptivity | Mass production is difficult; Immune response to disease needs to be further explored |
Exosomes and Cancer Vaccines
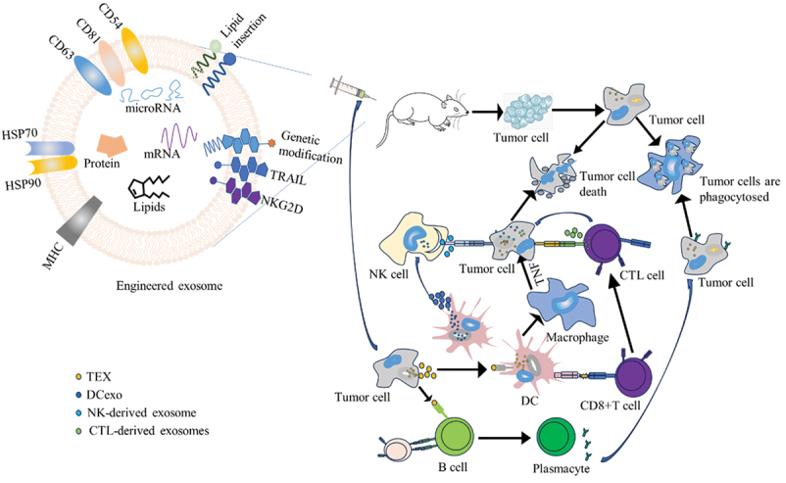 Fig. 1 (a) TEXs are modified to enrich tumor antigens, microRNAs, and immunostimulatory molecules on TEXs to enhance tumor cell death or to be killed by immune cells. (b) TEX is presented to CD8+ T cells by DC, ultimately leading to tumor cell death through cytotoxicity. (c) TEX activates the B cell response to produce antibodies, which eventually causes the tumor cells to be phagocytosed by macrophages. (d) TEX-loaded DCs activate macrophages, which participate in cytotoxicity by releasing TNF. (e) DCexo induces NK cell function and NK cell-derived exosome presents cytotoxic effects via perforin and FasL. (f) CTL cell-derived exosomes cause tumor cell death through TCR-MHC/antigen interactions.
Fig. 1 (a) TEXs are modified to enrich tumor antigens, microRNAs, and immunostimulatory molecules on TEXs to enhance tumor cell death or to be killed by immune cells. (b) TEX is presented to CD8+ T cells by DC, ultimately leading to tumor cell death through cytotoxicity. (c) TEX activates the B cell response to produce antibodies, which eventually causes the tumor cells to be phagocytosed by macrophages. (d) TEX-loaded DCs activate macrophages, which participate in cytotoxicity by releasing TNF. (e) DCexo induces NK cell function and NK cell-derived exosome presents cytotoxic effects via perforin and FasL. (f) CTL cell-derived exosomes cause tumor cell death through TCR-MHC/antigen interactions.
In cancer immunotherapy, tumor-derived exosomes (TEX) which are rich in tumor antigens can be presented by APC to induce T and B cell responses. Tumor-derived exosomes (TEX) have emerged as a promising cell-free therapeutic vaccine in cancer immunotherapy. TEXs are enriched with proteins such as major histocompatibility complex I and II (MHCI and MHCII), CD81, CD54, and CD63, which can be used for exosome binding and uptake by related proteins on DCs. Meanwhile, heat shock proteins are presented on TEX, including 71 kDa heat shock protein, 70 kDa heat shock protein 4, and HSP90 α & β. The heat shock protein (HSP) targets and activates antigen-presenting cells including dendritic cells (DCs), thus providing a natural link between the innate and adaptive immune responses. HSPs have powerful adjuvant capabilities that enhance the immunogenicity of TEXs and improve the effectiveness of cancer vaccines.
In addition to tumor-derived exosomes, dendritic cell-derived exosomes (DCexos) also have the potential to be cell-free therapeutic vaccines due to their resistance to tumor immunosuppression, significant bioavailability, and biostability. DCexos which are inert vesicles have a longer half-life in vivo and may be stored for a longer period. It is reported that DCexos are also more resistant to immunomodulation by the tumor or tumor microenvironment (TME) than DCs, thus supporting their clinical application as tumor vaccines.
Exosomes and COVID-19 Vaccines
At present, the outbreak of the COVID-19 pandemic has accelerated the research on EVs, especially the development of EV-based vaccines. As exosomes are essential intercellular communication molecules with immunogenicity, high biocompatibility, and inherent cargo-loading capacity, they offer new opportunities for the development of COVID-19 vaccines and may be crucial in restricting the COVID-19 pandemic.
Creative Bioarray Relevant Recommendations
| Service/Product Types | Description |
| Exosome Antibodies | Creative Bioarray offers a list of monoclonal/polyclonal antibodies against the common and disease-specific exosomal proteins. These antibodies are suitable for common applications including WB, ELISA, FC, IHC, ICC, IF, IP, etc. |
| Exosome Isolation Tools | Creative Bioarray aims to develop the best quality exosome isolation tools with optimized conditions to help our customers obtain pure exosomes with a higher yield. |
| Exosome Quantification | Creative Bioarray offers a range of options to meet most exosome quantitation demands. We provide reliable, and optimized tools at the most competitive price for quantitative analysis of exosomes in diverse biological samples such as plasma, urine, etc. |
RELATED PRODUCTS & SERVICES
Reference
- Dai Z, et al. (2024). "Exosome may be the next generation of promising cell-free vaccines." Hum Vaccin Immunother. 20 (1): 2345940.