How to Use Macrophages for Drug Delivery?
Bioactive Materials. 2024 Apr 23; 38: 55-72.
Authors: Guo Q, Qian ZM
INTRODUCTION
As a natural immune cell and antigen-presenting cell, macrophages have been studied and engineered to treat human diseases. Macrophages are well-suited for use as drug carriers because of their biological characteristics, such as excellent biocompatibility, long circulation, intrinsic inflammatory homing, and phagocytosis. Meanwhile, macrophages' uniquely high plasticity and easy re-education polarization facilitate their use as part of efficacious therapeutics for the treatment of inflammatory diseases or tumors.
Macrophages are the largest type of leukocytes, with an average diameter of 25 μm. This large size provides sufficient loading space for a variety of drugs with different physical and chemical properties and provides great convenience for modifying drugs. For example, cytoplasmic compartments provide sufficient internal space for hydrophilic drug loading, and the lipid bilayer of the cell membrane is particularly suitable for hydrophilic drug loading. Nevertheless, the low drug-loading capacity of cells remains a drawback of macrophage-based cell therapy.
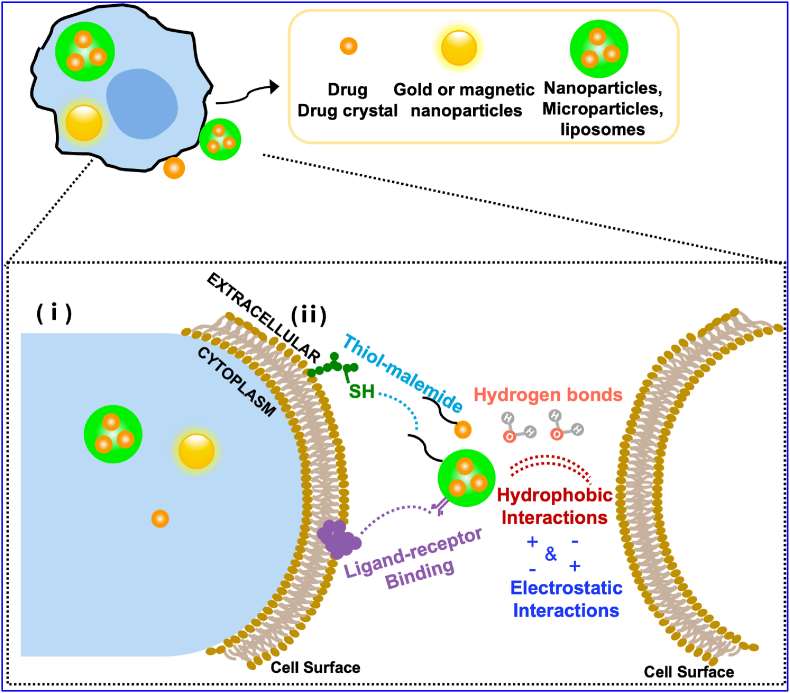 Fig. 1 Schematic illustration of different drug formulations-loading strategies for macrophage-based drug delivery systems.
Fig. 1 Schematic illustration of different drug formulations-loading strategies for macrophage-based drug delivery systems.
Loading Drugs into the Cytoplasmic Space of the Macrophages
Macrophages can internalize drugs or drug-loaded particles directly through phagocytosis. Loading drugs or particles into the cell cytoplasm provides multifaceted advantages. The half-life of the drug contained within macrophages is longer because the encapsulated drug is not rapidly eliminated by renal excretion or liver metabolism, is not immediately recognized by the immune system, and is not cleared by the endogenous reticuloendothelial system (RES). In addition to extending the circulatory half-life, macrophages can slow the release of drugs. Furthermore, encapsulation of drugs in the internal volume of cells is the simplest and most economical route and is also the preferred route for industrial production.
The amount of drug that macrophages can carry depends mainly on the phagocytosis effect. Most drugs that are loaded directly into macrophages are generally highly cytotoxic to macrophages. Hence, loading drugs into macrophages with the aid of nano or microcarriers rather than loading free drugs is an effective approach. In addition, nano- or micro-carriers can slow or delay the release of drugs from the cells before the macrophages reach their target, thereby maximizing the therapeutic effect of the drugs on the disease. The various physicochemical properties of the particles, in particular their size, shape, surface charge, hydrophobicity, and mechanical flexibility, determine the effectiveness of cellular uptake.
- Size. In terms of the properties of particles, particle size is considered to be the most critical factor. There is increasing evidence that large particles are phagocytized more efficiently by macrophages. Larger particles may condense into spherical structures within the cell membrane, possibly membrane-bound vesicles, which can be phagocytized within a short period. In addition, contact angle measurements showed that larger particles are more hydrophobic, which would provide evidence for higher macrophage uptake. Particles with smaller sizes may exhibit higher levels of exocytosis after being engulfed by macrophages.
- Surface charge. Surface charge also plays a key role in macrophage uptake. In general, charged particles are more rapidly engulfed by macrophages compared to neutral particles, possibly due to electrostatic interactions between particles and phagocytes. And positively charged nanoparticles accumulate to a greater extent in macrophages than negatively charged particles. However, in addition to dosage, surface charge, especially positive charge, maybe the most critical particle property in determining cytotoxicity.
- Shape. The shape of the particles also affects how they are internalized by macrophages. Long-chain nanomaterials such as filaments and nanorods are more efficient at evading mononuclear phagocyte systems (MPS) than traditional spherical particles and have a longer cycle time. In other words, spherical nanomedicines with the same curvature and contact points may be best suited for macrophage internalization.
- Surface coatings. Surface coatings are also thought to have an important effect on the phagocytosis of macrophages. Particles functionalized by mannans, non-specific complement opsonization, and specific antibody conditioning can be effectively internalized into macrophages through receptor-mediated endocytosis.
Loading Drugs onto the Surface of Macrophages
In addition to loading drugs inside macrophages, loading drugs onto the surface of macrophages is an alternative approach. The cell membrane is composed of rich chemical groups such as proteins, lipids, and carbohydrates, primarily charged or active groups that allow drugs or drug-loaded particles to be loaded onto the cell surface by covalent or non-covalent conjugation methods.
- Covalent methods typically utilize reactive chemical groups on the cell surface, such as amino groups on membrane proteins, thiol groups on cysteine residues, and glycosyl groups on glycoproteins or glycolipids. Among them, N-hydroxy succinimide ester is the most commonly used reactive group to form stable amide bonds between cell membranes and drugs or particles. In addition, orthogonal chemical groups can be artificially introduced onto cell membranes by treating cells with metabolic compounds such as azido-glycan and azido-phosphatidylcholine. This approach allows more than 100 nanoparticles with diameters in the range of 100-300 nm to be covalently linked to cell types commonly used in cell therapy without causing toxicity or affecting innate cellular functions. Covalent bonds provide the strongest link between macrophages and drugs, enhancing the stability of cell-drug interactions and avoiding the detachment and uptake of particles in non-target tissues such as the liver and spleen.
- Non-covalent methods rely on electrostatic interactions, hydrogen bonding, van der Waals forces, and hydrophobic interactions to load drugs or drug-loaded particles onto macrophage cell membranes. They require only minimal modification of cells and nanoparticles, providing that nanoparticles are either positively charged or sufficiently hydrophobic. The main advantage of this approach is that it is convenient and does not compromise important cellular functions. At the same time, the non-covalent methods are not strong enough to retain nanoparticles on the cell membrane and have relatively low stability due to the fluidity of the lipid bilayer. The attachment between particles and cells is difficult to control and can result in unpredictable behavior and detachment from the cell membrane due to blood flow-induced shear stress or cell-cell interactions in vivo.
Creative Bioarray Relevant Recommendations
| Service/Product Types | Description |
| Macrophages | We offer a wide range of macrophages. According to their polarization status, macrophages can be divided into M0, M1 (classically activated or pro-inflammatory), and M2 (alternatively activated or anti-inflammatory) macrophages. |
| Administration Routes and Biofluid Sampling | Creative Bioarray offers a variety of administration routes and collection of biological samples, as well as we can provide comprehensive bioanalytical package services. |
RELATED PRODUCTS & SERVICES
Reference
- Guo Q, Qian ZM. (2024). “Macrophage-based drug delivery: Key challenges and strategies.” Bioact Mater. 38: 55-72.