How to Handle Mycoplasma in Cell Culture?
The smallest prokaryotic microorganisms known as Mycoplasma lack cell walls and display high pleomorphism and were first discovered in 1898. Their sizes between 300 and 800 nanometers allow them to pass through standard cell culture filters which results in easy detection during cell culture procedures. Detecting mycoplasma can be difficult because cells often undergo multiple passages before the contamination becomes apparent. Research data indicates cell culture contamination rates can reach 35% which requires the development of robust detection and elimination techniques. The typical methods to detect contamination are microbial culture, fluorescent staining, immunostaining, PCR, electron microscopy and flow cytometry. The best practice involves implementing at least two detection methods together.
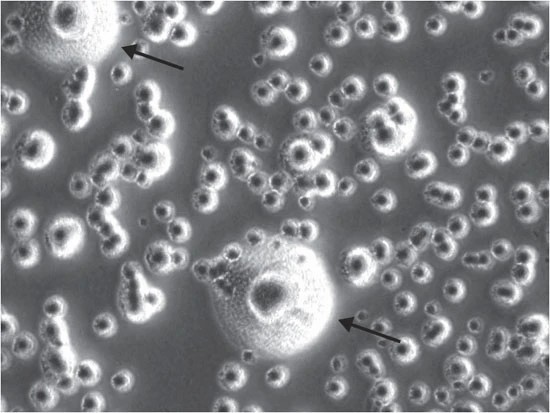 Fig. 1. Typical Mycoplasma colony, having the classic 'fried-egg' morphology (Young L, Sung J, et al., 2010).
Fig. 1. Typical Mycoplasma colony, having the classic 'fried-egg' morphology (Young L, Sung J, et al., 2010).
Table 1. Mycoplasma detection methods, their sensitivity, and advantages and disadvantages (Young L, Sung J, et al., 2010).
| Method | Sensitivity | Advantages | Disadvantages |
| Direct DNA stain (e.g., Hoechst 33258) | Low | Rapid, cheap | Can be difficult to interpret |
| Indirect DNA stain (e.g., Hoechst 33258) with indicator cells (e.g., 3T3) | High | Easy to interpret because contamination amplified | Indirect and thus more time-consuming |
| Broth and agar culture | High | Sensitive | Slow and may require expert interpretation |
| PCR | High | Rapid | Requires optimization |
| Nested PCR | High | Rapid | More sensitive than direct PCR, but more likely to give false positives |
| ELISA | Moderate | Rapid | Limited range of species detected |
| Autoradiography | Moderate | Rapid | Can be difficult to interpret if contamination is at low level |
| Immunostaining | Moderate | Rapid | Can be difficult to interpret if contamination is at low level |
Impacts of Mycoplasma Contamination
- Cell Proliferation and Metabolism: The presence of Mycoplasma contamination reduces cell proliferation rates and metabolic activity which results in slower cell growth and may cause cell death.
- Gene Expression and Chromosomal Abnormalities: Experimental results become unreliable because contamination modifies gene expression patterns and generates chromosomal abnormalities.
- Immune Response and Signaling Pathways: Mycoplasma contamination triggers cellular immune responses while also modifying signaling pathway activities, such as the phosphorylation of NF-kB and MAPK pathways.
- Metabolomics Changes: Contamination affects cell metabolomics, altering key metabolite levels, such as arginine and purine metabolism.
- Protein Degradation: Mycoplasma can degrade extracellular beta-amyloid (Aβ), affecting protein accumulation.
Strategies for Controlling Mycoplasma Contamination
The best approach to control mycoplasma contamination involves preventing its entry during the initial stages of cell culture. Methods to prevent mycoplasma introduction include:
1. Controlling Sources: Secure cell lines, media, and reagents from trusted cell banks and confirm their prior testing for mycoplasma and sterilization before acquiring them.
2. Strict Aseptic Inoculation Procedures: A sterile laboratory environment requires regular surface disinfection combined with sterile workbenches and strategies to prevent cross-contamination. Utilize sterile filters for media and reagents filtration to block mycoplasma transmission through aerosols.
3. Regular Testing: Regularly test cell cultures for mycoplasma to detect and address contamination promptly.
4. Implementing Strict Laboratory Management Protocols: Create and maintain strict laboratory management protocols that enforce aseptic cell culture handling while ensuring reagents remain sterile and waste is disposed of safely.
5. Using Antibiotics for Prevention: Preventive antibiotic treatment such as tetracyclines, macrolides, or quinolones should be carefully considered due to potential issues with antibiotic resistance.
When mycoplasma contamination has occurred, different strategies can be chosen based on the contamination severity and cell importance:
1. Using Mycoplasma Eradication Agents:
- The tolerance of different cells to mycoplasma eradication agents varies. Reduce concentration if cell growth slows or the condition deteriorates.
- Some eradication agents should not be mixed with antibiotics to avoid antagonistic effects.
- Consider using preventive mycoplasma eradication agents for valuable cells.
2. Physical Methods:
- Drug-Assisted Heat Treatment: Treat contaminated cells with antibiotics (e.g., doxycycline, streptomycin) and then incubate at 41°C for 18 hours. This method effectively kills mycoplasma but can be toxic to cells, so it must be used cautiously.
- Washing and Purification Method: This method reduces mycoplasma numbers through repeated centrifugation and washing. Specific operations include cell nutrition acclimation, selection of high-quality cell populations, cell washing, and repeated centrifugation. This method, combined with the suppressive action of sensitive antibiotics, can effectively eliminate mycoplasma.
3. Immuno-Response Elimination Methods:
- In Vivo Animal Inoculation: For important cell strains, contaminated tumor cells can be inoculated subcutaneously or intraperitoneally into animals of the same species, leveraging the animal’s immune system to eliminate mycoplasma. Extract the cells for culture after tumor cells continue to grow. This method is suitable for valuable or rare cell sources.
- In Vitro Phagocytosis: Extract macrophages from animal peritoneum and add them to contaminated cell cultures to clear mycoplasma through phagocytosis.
If the above methods fail to eliminate contamination, one may choose to restart the experiment or discard the contaminated cells and consumables.
Reference
Young L, Sung J, et al. Detection of Mycoplasma in cell cultures. Nat Protoc. 2010. 5(5):929-34.
Creative Bioarray Relevant Recommendations
| Products & Services | Description |
| Sterility Testing | Sterility testing of cell lines, media, in-process material and final products must be demonstrated during the manufacture of pharmaceuticals and medical devices. |

