How to Detect and Remove Endotoxins in Biologics?
Lipopolysaccharides (LPS) make up endotoxins which are crucial components of Gram-negative bacterial cell walls and they appear frequently in pharmaceutical ingredients medical devices and biological products. The sources of endotoxins include bacterial cell decomposition as well as contamination from production water and residues from biofilms on machinery. Endotoxins present serious pyrogenic risks because they trigger severe reactions such as fever or organ failure at minimal concentration levels and they interfere with biopharmaceutical operations by promoting protein aggregation and damaging cell cultures which results in reduced drug performance and safety problems. Endotoxin contamination in medical environments can provoke infusion reactions and dialysis complications while also causing infections from implanted medical devices which put patient lives at immediate risk. International pharmacopeias such as USP and EP together with medical device standards like ISO 23500 enforce stringent endotoxin control requirements during vaccine, monoclonal antibody, and gene therapy manufacturing processes with endotoxin levels needing strict regulation between 0.001 and 5 EU/mL as an essential quality standard.
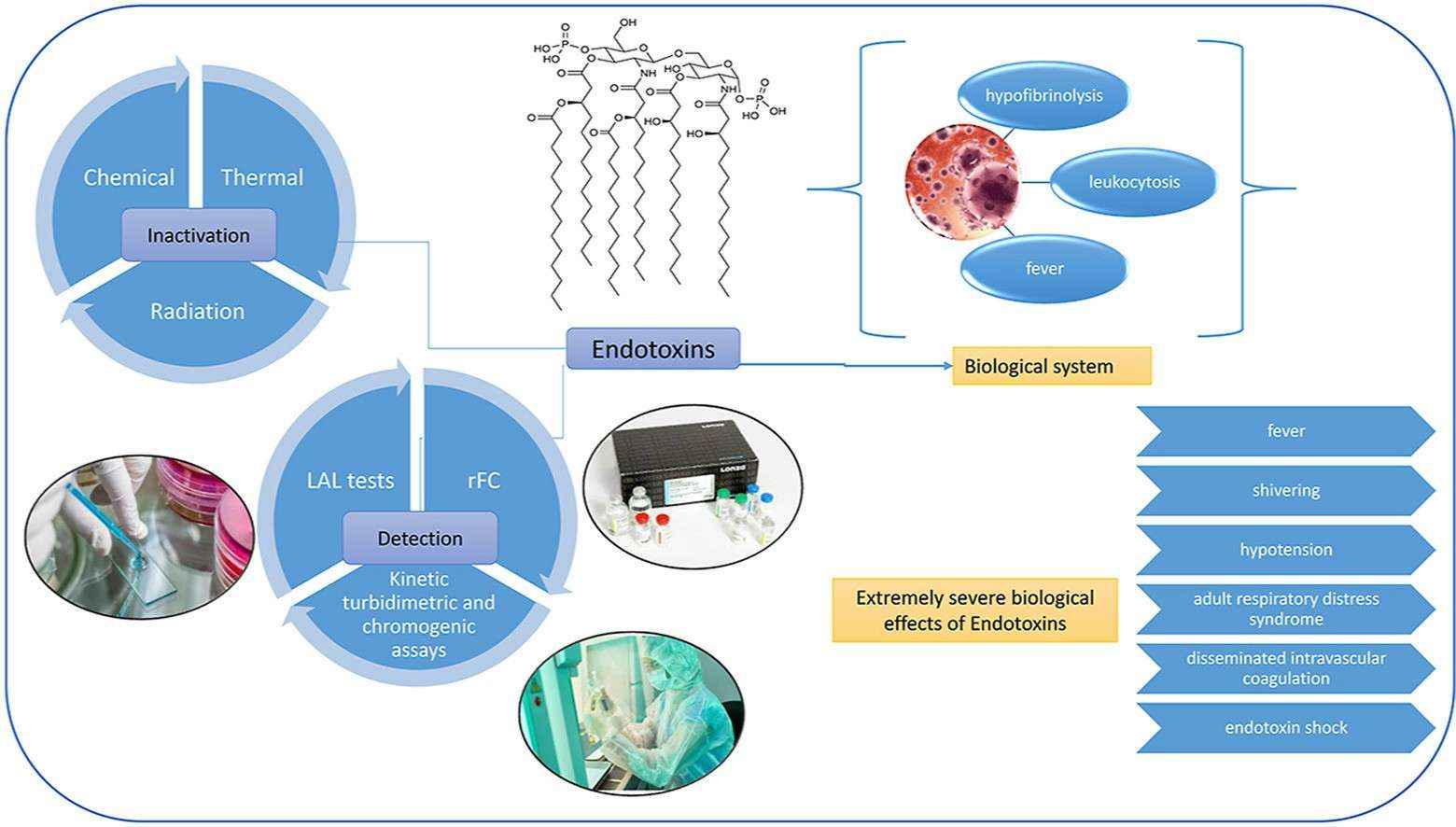 Fig. 1. Overview of endotoxins in biologics (Sheraba NS, Hesham A, et al. 2023).
Fig. 1. Overview of endotoxins in biologics (Sheraba NS, Hesham A, et al. 2023).
Endotoxin Detection Methods
Traditional methods
- Rabbit Pyrogen Test: The traditional rabbit pyrogen test assesses endotoxin presence through body temperature monitoring in rabbits but faces limitations due to its dependence on live animals and its requirement for large sample volumes which compromise both accuracy and sensitivity.
- Bovine Whole Blood Assay: The Bovine Whole Blood Assay identifies endotoxins by measuring PGE2 production in bovine leukocyte reactions. The method struggles with non-specific binding outcomes and difficulties obtaining bovine blood.
Modern methods
- Gel Clot: Observes gel formation upon reaction of endotoxins with Limulus amebocyte lysate.
- Turbidimetric: Quantifies using turbidity change from the reaction.
- Chromogenic: Utilizes chromogenic substrates for endotoxin detection.
- Recombinant C Factor Assay: Based on LAL principles, avoids false positives from non-specific sugar binding in LAL, with comparable costs.
- Monocyte Activation Test (MAT): Measures cytokines produced by monocytes when activated by endotoxins, similar to the chromogenic LAL assay.
Emerging technologies
- Electrochemical Techniques: Detect endotoxins through changes in electrical impedance upon contact with electrode-protein complexes.
- Fluorescence and Luminescence Techniques: Utilize bioluminescent reactions with pNA substrates for rapid detection.
- Surface Plasmon Resonance and Mass-based Technologies: Plasma biosensors track refractive index changes, while EMPAS sensors detect through high affinity with endotoxins.
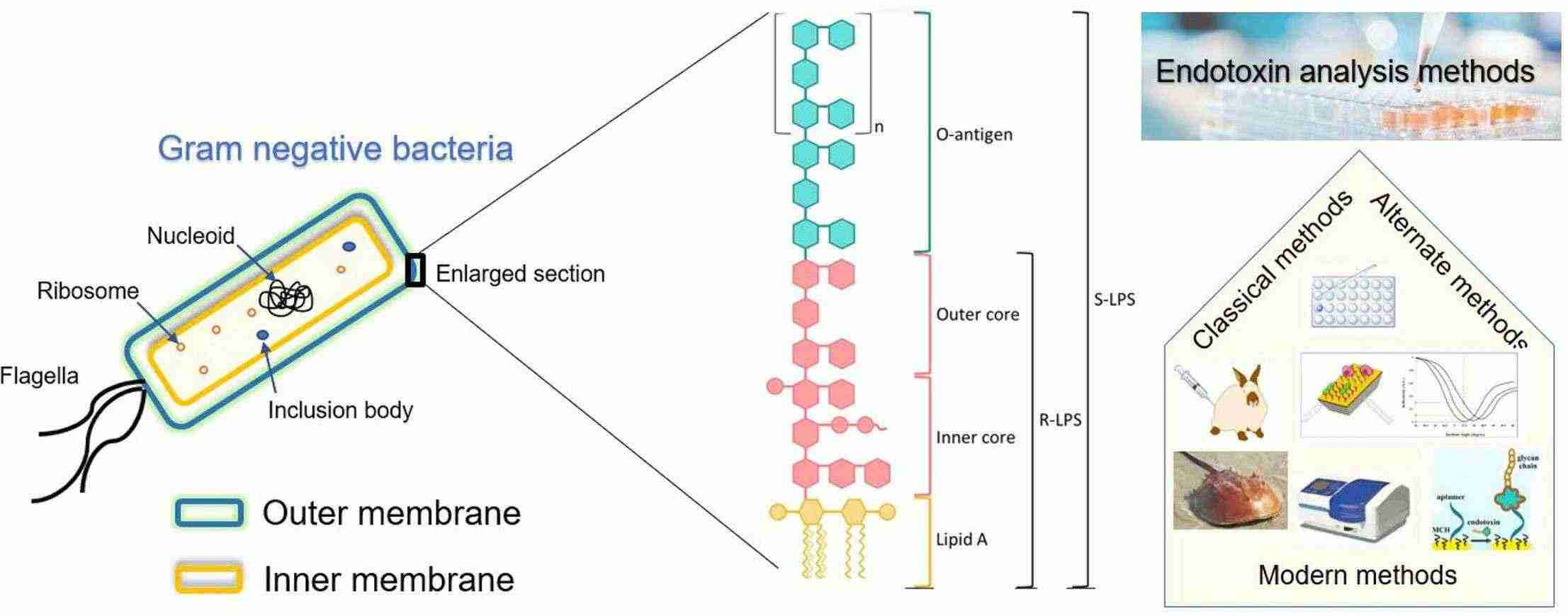 Fig. 2. Endotoxin Detection (Sondhi R, Adeniji T, et al. 2024).
Fig. 2. Endotoxin Detection (Sondhi R, Adeniji T, et al. 2024).
Factors Affecting Endotoxin Detection
- Sample Composition Interference: Polysaccharides, proteins, nucleic acids, metal ions, organic salts, and surfactants can affect results, as well as improper sample dilution.
- Detection Methods: Techniques like LAL assay vary in sensitivity to interference, e.g., dynamic turbidimetric methods require precise conditions to avoid disruptions.
- Reagents: Variability in LAL reagent sensitivity and storage impacts outcomes, as liquid form instability degrades response sensitivity.
- Environmental Conditions: Factors like temperature and pH affect endotoxin activity; e.g., endotoxins' heat resistance complicates removal, while water pH and salt concentration impact activity levels.
- Operational Precision: Accurate procedures are vital; incorrect handling can cause false positives or negatives.
Endotoxin Removal Methods
Physical methods
- Ultrafiltration: Uses fixed pore size membranes to separate large endotoxin micelles from small molecule proteins, with efficiency influenced by protein and detergent concentrations.
- Extraction: Employs solubility differences in immiscible fluids to separate endotoxins, using solvents like 1-octanol and Triton X-114; however, impacts on product yield must be managed.
- Activated Carbon Adsorption: Adsorbs large endotoxin molecules via hydrophobic interactions, more effective under weakly acidic conditions, suitable for low-molecular-weight solutions.
Chemical methods
- Ion Exchange Chromatography: Utilizes anion exchange ligands to bind endotoxins, removed by high-salt buffers, but efficacy may be limited with negatively charged proteins and DNA.
- Affinity Chromatography: Employs ligands like ε-poly-L-lysine for high-capacity binding; efficient protein recovery is achievable.
- Membrane Adsorption: Offers speed and reduced diffusion constraints compared to column methods; affinity ligands provide effective endotoxin adsorption.
Biodegradation pathways
- Phage Endolysin Cleavage: Enzyme targets Gram-negative outer membranes, cleaving peptidoglycan layers to hydrolyze LPS lipid A core.
- Engineered Phospholipase A1 (PLA1): Enhances affinity for LPS outer membranes, specifically hydrolyzing fatty acid chains.
- Synthetic Microbial Communities: Constructs gene-edited microbes to regulate LPS enzyme expression, achieving deacylation and polysaccharide chain oxidation.
Combining endotoxin removal methods maximizes efficiency. Process design must align with endotoxin characteristics and step-specific effectiveness, ensuring tailored approaches for diverse sample origins.
References
- Sheraba NS, Hesham A, et al., Advanced approaches for endotoxin detection and removal from snake antivenoms. Toxicon. 2023. 222:107003.
- Sondhi R, Adeniji T, et al., Chapter One - Advances in endotoxin analysis. Advances in Clinical Chemistry, 2024. 118:1-34.
Creative Bioarray Relevant Recommendations
| Products & Services | Description |
| Sterility Testing | Sterility testing of cell lines, media, in-process material and final products must be demonstrated during the manufacture of pharmaceuticals and medical devices. |

