Guidelines for Cell Banking to Ensure the Safety of Biologics
Proper cell banking is a fundamental requirement for the development and production of biologics, such as monoclonal antibodies, recombinant proteins, and cell-based therapies. The establishment of well-characterized and stable cell banks is essential to maintaining the integrity and consistency of the production process, ultimately safeguarding the quality and safety of the final biological product.
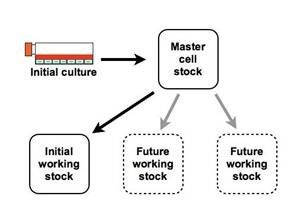 Fig.1 A two-stage approach using a master and working stock is more practical and flexible for cell banking. (Bhat KA, et al., 2022)
Fig.1 A two-stage approach using a master and working stock is more practical and flexible for cell banking. (Bhat KA, et al., 2022)
What Is Cell Banking?
Cell banking is a strategic approach to preserving well-characterized cell lines or master cell banks (MCBs) that serve as the foundation for the production of biologics. These MCBs are typically derived from a single, extensively tested, and fully characterized cell clone. The MCBs are then used to generate working cell banks (WCBs), the primary cell source for the actual manufacturing process.
Advantages of Cell Banking
The implementation of a robust cell banking system offers several key advantages in the biopharmaceutical industry:
- Consistent product quality. By maintaining a stable and well-characterized cell line, cell banking ensures consistent product quality and performance across multiple manufacturing batches, reducing the risk of process variability and product inconsistencies.
- Scalability and flexibility. The availability of multiple cell bank vials allows for scaling up production capacity as needed, without requiring the cell line for each new manufacturing campaign.
- Traceability and regulatory compliance. Comprehensive documentation and control of the cell banking process enable strict traceability, which is essential for regulatory compliance and ensuring the safety and efficacy of the final biological product.
- Mitigating contamination risks. Proper cell banking practices, including rigorous testing and monitoring, help identify and eliminate potential contaminants, minimizing the risk of microbial, viral, or genetic instability issues during production.
- Long-term stability. Cryopreservation techniques employed in cell banking allow for the long-term storage and preservation of cell lines, ensuring the availability of a reliable and consistent source of cells for future manufacturing needs.
Types of Cell Banks
Cell banks are categorized into different types based on their purpose and role in biotechnological research and manufacturing. Research and development cell banks (R&D CBs) are, as the name already anticipates, used for research purposes. The two primary types of cell banks in biotechnological manufacturing are the MCB and the WCB, each serving distinct functions in the preservation and utilization of cell lines. End-of-production cell lines, ultimately, are generated when the production has come to an end and are important for quality control and as a reference.
R&D CB
This cell bank acts as a foundation for the development of an MCB. As the name suggests, it is used for research and development purposes. It's a pre-GMP cell bank, meaning regulations are not as stringent compared with those for GMP-developed banks.
MCB
The MCB is the initial, extensively characterized, and documented cell line that serves as the foundation for all subsequent cell bank generations. The MCB is the most critical component of the cell banking process, as it ensures the genetic and phenotypic stability of the cell line.
WCB
The WCB is generated from the MCB and is the primary source of cells used for the actual production of biologics. The WCB is typically maintained in smaller aliquots, allowing for the efficient distribution and utilization of the cell line during manufacturing operations.
End-of-production cell bank (EOP CB)
This cell bank is created from vials of the WCB. The cell bank undergoes detailed analysis, including assessments of cell count, viability, shape, genetic structure (karyotyping), presence of contaminants like mycoplasma, and the existence of endotoxins. These cells act as a quality check for future use in research and development and can serve as references.
Cell Bank Manufacturing
Cell bank manufacturing is a crucial and systematic process that involves the expansion and cryopreservation of cells to establish reliable and reproducible cell banks. The process begins with the careful propagation of cells from the MCB or WCB. Under optimized cell culture conditions, cells are grown to generate a sufficient quantity for future use. This step is essential to maintain the genetic stability and functionality of the cells, ensuring consistency and reproducibility in downstream applications.
Once the cells have been expanded, they undergo cryopreservation in individual vials or containers like small single-use bags. Cryoprotectants are added to protect the cells from damage during the freezing and thawing processes.
Sterility assurance plays a crucial role in maintaining the integrity of the cell lines. Adherence to aseptic techniques and cleanroom environments ensures that cell banks remain free from contaminants and microbial growth. Additionally, strict regulations such as cGMP regulations ensure that the cell banking process meets the required quality standards and safety protocols.
Cell Bank Characterization
Comprehensive characterization of cell banks is crucial to ensure the safety and consistency of the biological products. The characterization process typically includes the following:
- Cell line identity. Techniques such as DNA fingerprinting, isoenzyme analysis, and karyotyping are employed to confirm the identity of the cell line.
- Cell line viability. This testing is crucial to maintaining the consistency of the manufactured biological products. Cell line viability refers to the ability of the cells to remain alive and functional over time.
- Genetic stability. Stability refers to the ability of the cells to maintain their genetic and phenotypic characteristics over time. Analyses of the cell line's genetic profile, including chromosome number and structure, are performed to monitor for any genetic drift or instability over time.
- Phenotypic characterization. The characterization of cell lines before banking is essential in drug manufacturing processes. One validated technique used for phenotypic characterization and derivation of clonal cell lines is the FACS (fluorescence-activated cell sorting) technique. It separates specific cell populations from a heterogeneous cell sample based on their surface markers.
- Microbial and viral testing. Extensive testing for the presence of mycoplasma, bacteria, fungi, and viruses is conducted to ensure the absence of potential contaminants.
Maintenance of Cell Banks
Proper maintenance of cell banks is crucial to preserve the integrity and stability of the cell lines. Key aspects of cell bank maintenance include:
- Cryopreservation and storage. Cell banks are typically cryopreserved in liquid nitrogen or ultra-low temperature freezers to ensure long-term stability and viability.
- Controlled access and inventory management. Strict protocols are in place to monitor and control access to the cell banks, maintaining a comprehensive inventory system to ensure traceability.
- Periodic testing and monitoring. Routine testing and monitoring of the cell banks are conducted to verify the continued stability, purity, and functionality of the cell lines.
Creative Bioarray Relevant Recommendations
| Product/Service Types | Description |
| Cell Lines | Creative Bioarray provides the world's most comprehensive list of cells and has realized that animal and human primary cells, tumor cell lines, continuous (immortalized) cell lines, and tissues are very important to the biopharmaceutical industry and to biomedical research as reagents, therapeutic modalities, and as proxy materials. |
| Cell Line Testing and Assays | We have performed thousands of cell line testing services for clients in biopharma under cGMP and validated to current ICH guidelines. In addition, QC testing of cell lines, media, in-process material, and final products has been demonstrated during the manufacture of pharmaceuticals and medical devices by our experts. |
Reference
- Bhat KA, et al. (2022). "Advances in Nematode Identification: A Journey from Fundamentals to Evolutionary Aspects." Diversity. 14 (7), 536.

