A Brief Overview of Cellular Immortality Technologies
Life (Basel). 2024 Mar 21; 14 (3): 417.
Authors: Chalak M, Hesaraki M, Mirbahari SN, Yeganeh M, Abdi S, Rajabi S, Hemmatzadeh F.
INTRODUCTION
Numerous biotechnological approaches have been employed to manipulate the cellular genome to acquire immortalized cell lines. Nonetheless, the foremost and extensively applied techniques for achieving this involve the introduction of viral oncoproteins and telomerase reverse transcriptase (TERT). The immortality conferred by viral oncoproteins is intricately linked to the deactivation of cell cycle-regulating proteins (such as p16, p14, p21, p53, and Rb). Through this pathway, viral oncoproteins can induce the deactivation of tumor suppression mechanisms and may even trigger the expression of telomerase.
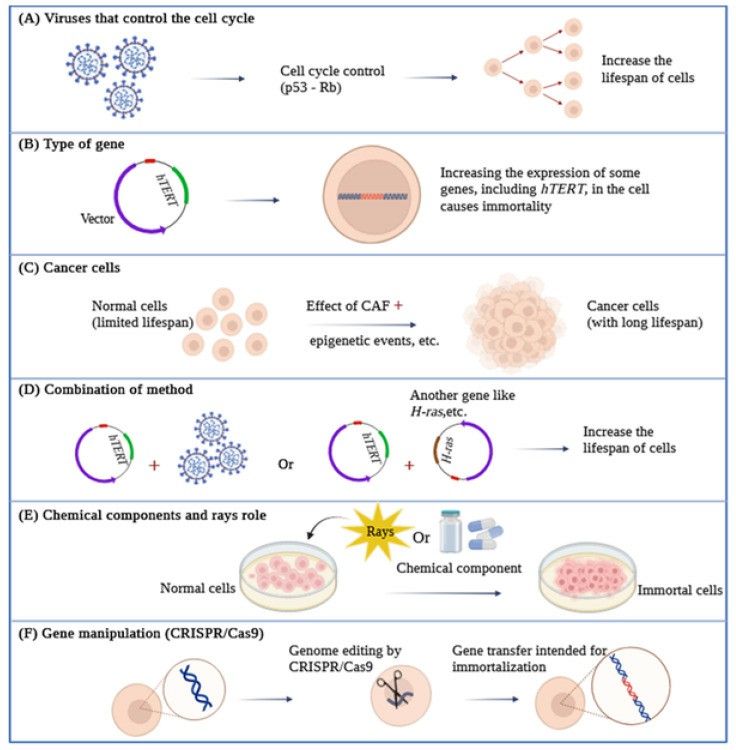 Fig. 1 An overview of various types of cell immortalization methods.
Fig. 1 An overview of various types of cell immortalization methods.
Viral Genes Can Control the Cell Cycle
As viruses rely on the ability to replicate within living organisms to survive, they can manipulate or accelerate the cellular cycle for their benefit. One of the methods to achieve this is through targeting the expression of Rb and p53 proteins. Viral oncogenes are also able to disable pRb and p53, thus overcoming the M1 barriers, which inhibit the growth and replication of natural cells, and significantly increase the lifespan of cells.
- Simian Virus 40. The Simian Virus 40 (SV40) encodes two proteins, Large T antigen (LT) and Small T antigen (ST), which help induce virus-associated tumors. SV40 T antigen is currently used for cell transfection in different types of cells and can generate immortal cell lines by binding and disabling p53 and Rb proteins. Both of these proteins have evolved to specifically target crucial cellular regulators and modify their functions. The Large T antigen, for instance, targets various known proteins encompassing 708 amino acids. These include three members of the retinoblastoma protein family (pRb, p107, and p130), as well as members of the Cap-binding protein (CBP) adaptor protein family, such as p300 and p400, along with the tumor suppressor protein p53. On the other hand, the Small T antigen affects the activity of the pp2A phosphatase and activates the cyclin A promoter. Notably, the LT protein plays a central role in conferring SV40's extended lifespan, primarily due to its capability to interact with growth suppressors like pRb and p53 and suppress the p53 pathway.
- Human Papillomavirus (HPV). HPV is a small, double-stranded DNA virus that infects mucosal and cutaneous epithelial tissues. High-risk strains, including HPV-8/16/18/31, cause malignant lesions, while low-risk strains, including HPV-6/11, cause benign warts and lesions. The E6 and E7 proteins, encoded by high-risk strains such as HPV-16/18, are classified as oncoproteins. When used in immortalization, E6 activates telomerase and accelerates the degradation of p53 by proteasome S26, while E7 can inactivate Rb by preventing the binding between pRb and the transcription factor E2F. For example, Trakarnsanga and colleagues achieved the immortalization of early adult erythroblasts by employing HPV-16 oncoproteins. This led to the creation of stable cells capable of producing red blood cells.
- Human T-Lymphotropic Virus (HTLV). HTLV is a human RNA retrovirus that causes leukemia and adult T-cell lymphoma. There are two types, HTLV-1 and HTLV-2, which can infect lymphocytes under laboratory conditions, although clinically, HTLV-2 is less pathogenic than HTLV-1. Types 1 and 2 encode for the Tax1 and Tax2 proteins, respectively, which are necessary for the infection of human T-cells by the associated viruses. Due to the greater pathogenic power of HTLV-1, the number of growth-induced cells by Tax2 was much greater than the cells induced by Tax1, and the activity of Tax2 was far higher than Tax1.
- Adenoviruses. Adenoviruses are common DNA viruses found in animals and humans and are often observed in adults and children. The 12S E1A gene product from adenovirus, belonging to the oncoprotein class, can establish primary cells as cell lines. It is encoded by two exons. Extensive mutational studies have revealed that four specific regions of the 12S E1A gene, derived from both exons, play a critical role in prolonging the lifespan of primary epithelial cells. While the expression of two of these regions is essential for activating quiescent cells and initiating the cell cycle, it alone cannot confer immortality or extend their lifespan in culture. These two regions are encoded by exon 1. The third region within exon 1, whose function remains unidentified, is also indispensable for this process. Moreover, these three regions are crucial for cooperating with 12S and an activated ras gene in triggering tumor formation. The fourth region is necessary for sustaining the proliferation of cells, prolonging their lifespan in culture, and prompting autocrine growth factor production. Cells immortalized by both wild-type 12S and its mutant variants maintain their epithelial characteristics, and they continue to express intermediate filament proteins like keratin and vimentin.
- Epstein–Barr Virus (EBV). EBV is a double-stranded DNA virus that infects B lymphocytes. Immortalizing B cells is an effective method for inducing the long-term growth of some human B cells in laboratory conditions. This virus can immortalize cells and convert them into lymphoblastoid cell lines that carry EBV. These cells effectively induce specific T-cell responses against EBV in laboratory conditions due to the presentation of viral antigens. It has been shown that EBNA-2 is genetically essential for B cell immortalization by EBV. Experiments have shown that EBNA-2 affects the accumulation of viral and cellular RNAs, and LMP may also be necessary for immortalization as it can affect the growth properties of human lymphoid and epithelial cells. EBNA-1 may also be necessary for the immortalization of a B cell for EBV, as it appears to be necessary for the maintenance of viral DNA replication in the replicating cell population.
Overexpression of Specific Genes for Immortalization
- The HOX Gene Family and Lhx2. The overexpression of the HOX11 and TLX1 genes can lead the hematopoietic precursor cells, mouse fetal liver, or bone marrow toward immortalization. A wide range of hematopoietic cell types have been immortalized using these methods, including erythroid, megakaryocytic, monocytic, myelocytic, and multipotent cells. Studies have also shown that myelomonocytic, megakaryocytic, and mast cell progenitor immortalization in mice can be achieved using the Hox-2.4 gene. It has also been reported that the expression of the HOXa9 gene can immortalize a promyelocyte.
- c-Myc Gene Expression. Another set of genes employed in the immortalization of cells belongs to the myc family. This family encompasses a group of oncogenes: c-Myc, N-Myc, L-Myc, and B-Myc. The expression of c-Myc is predominantly observed in actively proliferating cells, whereas N-Myc and L-Myc play roles in differentiation processes. Among these oncogenes, the c-Myc gene has been the subject of the most extensive studies. It is worth noting that there is an association between p53 and c-Myc, as the Myc signaling pathway governs both apoptosis and cell immortalization, with the latter being contingent on the status of p53.
- CDK4 Gene Expression. CDK4 expression, along with increased hTERT gene expression, has been used for the immortalization of human bronchial epithelial cells. Additionally, CDK4 expression, along with cyclin D1 and increased telomerase activity, has been used for the immortalization of human myogenic cells derived from healthy and diseased muscles.
- TERT Gene Expression. As mentioned, the risk of oncogenic integration into chromosomes still raises various safety concerns when using an oncogenic transferable agent in host cells. Using hTERT as a means of achieving immortalization through less phenotypic/karyotypic alterations has been proposed. hTERT is a key determinant of human telomerase enzyme activity. It comprises 16 exons and 15 introns and spans approximately 35 kb. Human primary cells have been immortalized by increasing hTERT expression in fibroblast, choroidal melanocytes, endothelial cells, dermal keratinocytes, mammary epithelial cells, osteoblasts, and pancreatic cells. Some important features observed in several types of immortalized cells by increasing hTERT expression include that they do not go towards malignancy, cell cycle control remains normal p53 and pRb checkpoints remain active, contact inhibition is still normal, cells require growth factors for proliferation, and cell karyotyping remains normal and does not show extensive changes.
Cancer Cells and Immortalization
Cell immortalization is a crucial stage in tumorigenesis where cells can overcome aging and critical situations. Along this path, cells become immortal naturally and can be isolated. Tissue samples are acquired through a biopsy, followed by a process of separation, where various cell types are isolated and assessed for their proliferation potential and unique characteristics.
However, in the process of the cell becoming cancerous, one of the contributing factors is cancer-associated fibroblasts or CAFs. CAFs are the dominant stroma surrounding the tumor. Studies have shown that CAFs can promote tumor growth, angiogenesis, resistance to chemotherapy and metastasis, and enhance cancer progression, thereby naturally directing cells toward immortality. A notable point in this regard is that the primary CAFs separated from human carcinomas have been shown to remain active even in laboratory conditions for a long period.
The expression of miR-200 is also concurrent with increased expression of the DNMT3B gene in CAFs. DNMT3B is not only a direct target of miR-221 but also affects miR-200b/c and regulates their expression in CAFs. On the other hand, miR-141 inhibition in these cells increases the expression of transcription factor 12 (TCF12) to facilitate the growth of breast cancer cells through CXCL12 secretion in CAFs, which leads to increased c-Myc and cyclin D1 expression in breast cancer cells, thus allowing these cells to undergo immortality through c-Myc activation.
Chemical Components and Rays Role in Immortalization
Cells can be immortalized through exposure to radioactive factors and chemical carcinogens. Certain chemicals, some of which are carcinogenic, can contribute to cell immortalization. These chemicals are categorized based on their carcinogenic potential. Each carcinogen can be further grouped based on its mode of action into genotoxic carcinogens or non-genotoxic carcinogens. Genotoxic carcinogens are substances or agents that directly initiate carcinogenesis by interacting directly with DNA, leading to DNA damage and chromosomal abnormalities that can be detected through genotoxicity testing. On the other hand, NGCs are agents capable of inducing cancer through a secondary mechanism, often as a result of their indirect impact on DNA. They can alter signal transduction pathways or gene expression. The GCs can be detected using genotoxicity testing, which detects changes to the cell at the molecular and cellular levels. These changes include mutations in genes, DNA strand breaks, the formation of DNA adducts, chromosomal aberrations, and aneuploidy.
Physical carcinogens (such as ionizing radiation) are also powerful immortalization agents with different mechanisms and frequencies in rodents and human cells]. For example, X-rays, neutrons, and gamma rays produce immortal clones in SHD cells. Conversely, the immortalization of human mammary cells through ionizing radiation is a relatively rare occurrence. Similarly, methyl sulfate, a powerful clastogen, is an efficient immortalizing carcinogen in mammalian SDH cells and Chinese hamster cells and has a similar mode of action to that of ionizing radiation.
Furthermore, the carcinogenic strength of basic aliphatic alkylating agents like alkylnitrosamides and alkylmethanesulfonates is directly associated with their capacity to modify the comparatively less reactive oxygen atoms in DNA, notably the O6 atom of guanine. In addition, data suggest that acetaminophen activates telomerase, which could lead to the immortalization of cells. However, there are also data indicating that acetaminophen can inhibit CDK4 and CDK2, thus imposing a cell cycle checkpoint at G1 and effectively blocking cellular proliferation.
Creative Bioarray Relevant Recommendations
| Service/Product Types | Description |
| Cell Immortalization Service | Creative Bioarray is offering cell immortalization services. Based on our experienced scientist team and elaborate technical platforms, we have been able to successfully immortalize cells from any species and any tissue with the function you need. |
| Epigenetic Induction of Cell Growth Service (Non-Viral Cell Immortalization Method) | Creative Bioarray is offering epigenetic induction of cell growth service for cell immortalization. With the Epigenetic Approach, we have established Immortalized Chicken Hepatocytes, Immortalized Monkey Hepatocytes, Immortalized Dog Hepatocytes, and Immortalized Human T Cells, which primary cells are very difficult to immortalize in traditional ways. |
RELATED PRODUCTS & SERVICES
Reference
- Chalak M, et al. (2024). "Cell Immortality: In Vitro Effective Techniques to Achieve and Investigate Its Applications and Challenges." Life (Basel). 14 (3): 417.