- You are here: Home
- Disease Models
- Oncology Models
- Humanized Mouse Models
- Humanized PBMC Mouse Models
- hPBMC-T Cell Reconstitution Model
Disease Models
- Oncology Models
-
Inflammation & Autoimmune Disease Models
- Rheumatoid Arthritis Models
- Glomerulonephritis Models
- Multiple Sclerosis (MS) Models
- Ocular Inflammation Models
- Sjögren's Syndrome Model
- LPS-induced Acute Lung Injury Model
- Peritonitis Models
- Passive Cutaneous Anaphylaxis Model
- Delayed-Type Hypersensitivity (DTH) Models
- Inflammatory Bowel Disease Models
- Systemic Lupus Erythematosus Animal Models
- Asthma Model
- Sepsis Model
- Psoriasis Model
- Atopic Dermatitis (AD) Model
- Scleroderma Model
- Gouty Arthritis Model
- Carrageenan-Induced Air Pouch Synovitis Model
- Carrageenan-Induced Paw Edema Model
- Experimental Autoimmune Myasthenia Gravis (EAMG) Model
-
Cardiovascular Disease Models
- Surgical Models
- Animal Models of Hypertension
- Venous Thrombosis Model
- Atherosclerosis model
- Cardiac Arrhythmia Model
- Hyperlipoidemia Model
- Doxorubicin-induced Heart Failure Model
- Isoproterenol-induced Heart Failure Model
- Arterial Thrombosis Model
- Pulmonary Arterial Hypertension (PAH) Models
- Heart Failure with Preserved Ejection Fraction (HFpEF) Model
-
Neurological Disease Models
- Alzheimer's Disease Modeling and Assays
- Seizure Models
- Parkinson's Disease Models
- Ischemic Stroke Models
- Acute Spinal Cord Injury (ASCI) Model
- Traumatic Brain Injury (TBI) Model
- Hypoxic-Ischemic Encephalopathy (HIE) Model
- Tourette Syndrome (TS) Model
- Amyotrophic Lateral Sclerosis (ALS) Model
- Huntington's Disease (HD) Model
- Intracerebral hemorrhage (ICH) Models
- Pain Models
- Metabolic Disease Models
- Liver Disease Models
- Rare Disease Models
- Respiratory Disease Models
- Digestive Disease Models
-
Urology Disease Models
- Cisplatin-induced Nephrotoxicity Model
- Unilateral Ureteral Obstruction Model
- 5/6 Nephrectomy Model
- Renal Ischemia-Reperfusion Injury (RIRI) Model
- Diabetic Nephropathy (DN) Models
- Passive Heymann Nephritis (PHN) Model
- Adenine-Induced Chronic Kidney Disease (CKD) Model
- Kidney Stone Model
- Doxorubicin-Induced Nephropathy Model
- Orthopedic Disease Models
- Ocular Disease Models
- Skin Disease Models
- Infectious Disease Models
hPBMC-T Cell Reconstitution Model
Are you striving to propel your preclinical endeavors forward and initiate in vivo evaluations of efficacy, safety, and toxicity within a comprehensive framework? Creative Bioarray presents a meticulously crafted hPBMC-T Cell Reconstitution Model, ideally suited for immune-oncology research endeavors. This groundbreaking model harnesses the potential of immunodeficient mice and integrates the infusion of vital peripheral blood mononuclear cells (PBMCs) sourced from healthy human donors. Following a 10 to 14-day reconstitution period post-infusion, the mice develop a human-like immune system, enabling them to be seamlessly coupled with both Cell Line Derived Xenograft (CDX) and Patient Derived Xenograft (PDX) models. This integration facilitates a thorough assessment of the antitumor efficacy of your test compounds, offering unparalleled insights into their potential therapeutic value and clinical applicability.
Applications
hPBMC-T Cell Reconstitution Model represents a groundbreaking advancement in the realm of immunology, enabling the successful establishment and maintenance of functional human immune systems within a controlled environment. By enabling the testing of novel therapeutic approaches within these humanized models, researchers can more accurately predict their efficacy and safety in clinical settings, ultimately paving the way for more effective and targeted cancer treatments.
Key Advantages
- High Success Rate and Reliable Reconstitution
hPBMC-T Cell Reconstitution Model boasts a success rate exceeding 85% and feature a stable reconstruction process, ensuring reliable experimental data and a strong foundation for research.
- Precise Timing for Tumor Cell Inoculation
We have improved the PBMC reconstruction process, allowing for the inclusion of animals in studies within 10-14 days post-PBMC inoculation. This precision helps researchers time tumor cell inoculation effectively, facilitating concurrent growth and addressing limited dosing windows.
- Compatible with Most of Tumor Cells
hPBMC-T Cell Reconstitution Model is compatible with 400+ CDX models and 400+ PDX models.
- Extended Dosing Window
By optimizing our protocols, we have extended the dosing window to over three weeks, providing flexibility for immune tumor research and enabling deeper exploration of immune-tumor interactions and treatment strategies.
Example Data
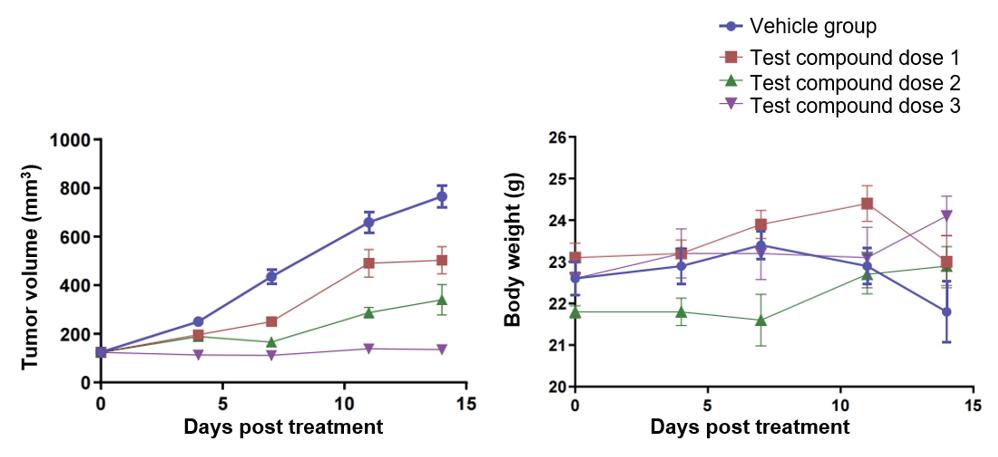 Fig. 1 Effects of the test compound on tumor volume and body weight in NCI-H292 tumor-bearing hPBMC-T reconstitution mice.
Fig. 1 Effects of the test compound on tumor volume and body weight in NCI-H292 tumor-bearing hPBMC-T reconstitution mice.
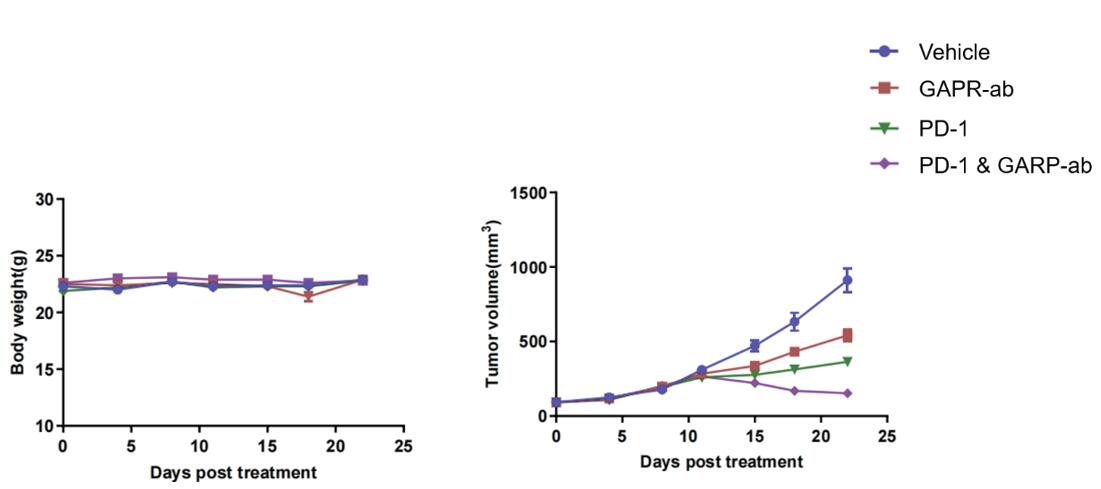 Fig. 2 Effects of immune checkpoint inhibitors on body weight and tumor volume in JeKo-1 tumor-bearing hPBMC-T reconstitution mice.
Fig. 2 Effects of immune checkpoint inhibitors on body weight and tumor volume in JeKo-1 tumor-bearing hPBMC-T reconstitution mice.
Quotation and Ordering
Creative Bioarray boasts a team of highly skilled scientists who have established a comprehensive platform for a wide range of rodent tumor models. Our goal is to support our clients in the discovery of new anti-tumor drugs and to facilitate any research endeavors in the field of oncology. If you are interested in our services, please feel free to contact us at any time or submit an inquiry to us directly.
For research use only. Not for any other purpose.

