- You are here: Home
- Disease Models
- Oncology Models
- Humanized Mouse Models
- Humanized PBMC Mouse Models
- hPBMC Donor Screening & QC Platform
Disease Models
- Oncology Models
-
Inflammation & Autoimmune Disease Models
- Rheumatoid Arthritis Models
- Glomerulonephritis Models
- Multiple Sclerosis (MS) Models
- Ocular Inflammation Models
- Sjögren's Syndrome Model
- LPS-induced Acute Lung Injury Model
- Peritonitis Models
- Passive Cutaneous Anaphylaxis Model
- Delayed-Type Hypersensitivity (DTH) Models
- Inflammatory Bowel Disease Models
- Systemic Lupus Erythematosus Animal Models
- Asthma Model
- Sepsis Model
- Psoriasis Model
- Atopic Dermatitis (AD) Model
- Scleroderma Model
- Gouty Arthritis Model
- Carrageenan-Induced Air Pouch Synovitis Model
- Carrageenan-Induced Paw Edema Model
- Experimental Autoimmune Myasthenia Gravis (EAMG) Model
-
Cardiovascular Disease Models
- Surgical Models
- Animal Models of Hypertension
- Venous Thrombosis Model
- Atherosclerosis model
- Cardiac Arrhythmia Model
- Hyperlipoidemia Model
- Doxorubicin-induced Heart Failure Model
- Isoproterenol-induced Heart Failure Model
- Arterial Thrombosis Model
- Pulmonary Arterial Hypertension (PAH) Models
- Heart Failure with Preserved Ejection Fraction (HFpEF) Model
-
Neurological Disease Models
- Alzheimer's Disease Modeling and Assays
- Seizure Models
- Parkinson's Disease Models
- Ischemic Stroke Models
- Acute Spinal Cord Injury (ASCI) Model
- Traumatic Brain Injury (TBI) Model
- Hypoxic-Ischemic Encephalopathy (HIE) Model
- Tourette Syndrome (TS) Model
- Amyotrophic Lateral Sclerosis (ALS) Model
- Huntington's Disease (HD) Model
- Intracerebral hemorrhage (ICH) Models
- Pain Models
- Metabolic Disease Models
- Liver Disease Models
- Rare Disease Models
- Respiratory Disease Models
- Digestive Disease Models
-
Urology Disease Models
- Cisplatin-induced Nephrotoxicity Model
- Unilateral Ureteral Obstruction Model
- 5/6 Nephrectomy Model
- Renal Ischemia-Reperfusion Injury (RIRI) Model
- Diabetic Nephropathy (DN) Models
- Passive Heymann Nephritis (PHN) Model
- Adenine-Induced Chronic Kidney Disease (CKD) Model
- Kidney Stone Model
- Doxorubicin-Induced Nephropathy Model
- Orthopedic Disease Models
- Ocular Disease Models
- Skin Disease Models
- Infectious Disease Models
hPBMC Donor Screening & QC Platform
Creative Bioarray proudly presents our state-of-the-art hPBMC Donor Screening & Quality Control (QC) Platform, a cornerstone of our commitment to excellence in immune system reconstruction for humanized mouse models. This innovative platform is meticulously designed to ensure the highest success rates for immune reconstruction and to extend the dosing window, crucial factors for the reliability and effectiveness of preclinical studies.
Our hPBMCs undergo a rigorous internal screening process, ensuring that each batch of PBMCs is pre-validated in vivo for optimal performance in your experiments. This stringent quality control not only guarantees a high success rate for immune system reconstruction but also secures an extended dosing window of up to three weeks, providing you with a robust and reliable research environment.
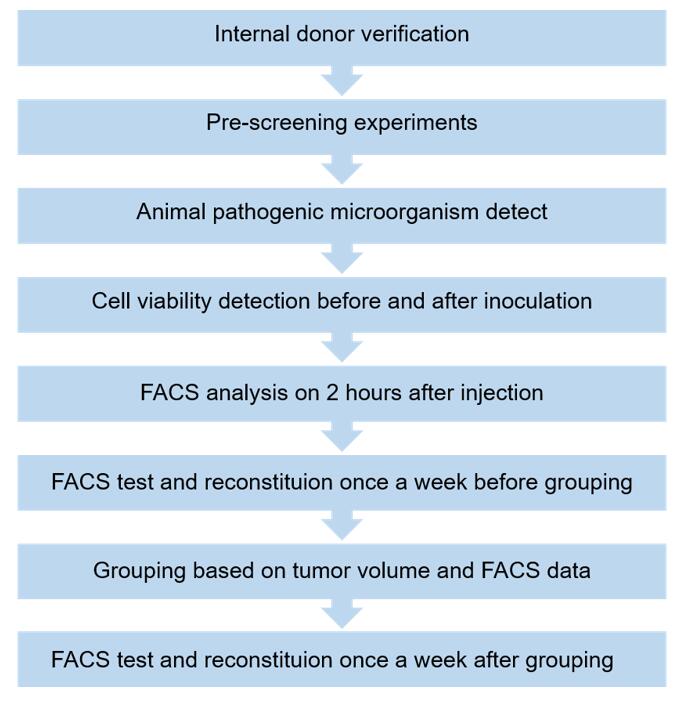 Fig. 1 Workflow of the hPBMC Donor Screening & QC Platform.
Fig. 1 Workflow of the hPBMC Donor Screening & QC Platform.
Why hPBMC Donor Screening & QC is Essential?
Human PBMCs are sourced from healthy donors, each exhibiting unique capabilities in terms of PBMC reconstruction, tumor cell antagonism, and response to immunotherapy drugs. The versatility of the PBMC model is a significant advantage, as it supports a wide range of tumor cell types, offering a diverse array of tumor-end targets to meet the efficacy testing requirements for various immunotherapies. However, the variability in antagonistic capabilities between different tumor cells and PBMCs poses a challenge: when PBMC donors are changed, historical data often lacks significant relevance to new donors. Consequently, most PBMC projects necessitate donor screening to identify donors compatible with the candidate tumor cells, with donor selection based on demonstrated efficacy.
Criteria of hPBMC Donor Screening
- hCD45+ Cell Proportion: After 14 days post-inoculation, the proportion of hCD45+ cells in peripheral blood should be at least 10%.
- Graft-versus-Host Disease (GvHD) Onset: The onset of GvHD must occur at 5 weeks or later, with no more than 10% weight loss.
Advantages
- Stable hPBMC reconstitution and compatibility: Over 90% of human tumor cells can be selected for reconstruction from three donors.
- Comprehensive pre-screening platforms: Offering both in vivo and in vitro pre-screening platforms to meet diverse customer needs.
- Robust PBMC donor reserves: Large-scale PBMC donor backup ensures stable donor use throughout consecutive projects.
Quotation and Ordering
Creative Bioarray stands as the preferred partner for industry leaders in pursuit of innovative solutions for immunotherapy research, thanks to our unwavering commitment to quality and innovation. Our hPBMC Donor Screening & QC Platform is designed to elevate your research to unprecedented levels, accelerating your journey towards scientific breakthroughs. If you are interested in our services, please do not hesitate to contact us at any time or submit an inquiry to us directly.
For research use only. Not for any other purpose.

