Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
Mouse iPSC Line
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The mouse iPSC Line represents pluripotent stem cells created by transforming mouse somatic cells like embryonic fibroblasts (MEFs). Japanese scientist Shinya Yamanaka's team developed this technique in 2006. They successfully induced these cells to become pluripotent stem cells with embryonic stem cell (ESC) characteristics by introducing four transcription factors: Oct4, Sox2, Klf4, and c-Myc. MEFs are a common starting cell type due to their ease of access, culture, and capacity for extensive proliferation. It is possible to reprogram other somatic cell types including hematopoietic stem cells, mesenchymal stem cells and others.
Mouse iPSCs show extensive unlimited proliferation potential and maintain their undifferentiated state throughout extended in vitro culture periods. These cells demonstrate standard pluripotent stem cell characteristics by differentiating into multiple cell types such as neurons, cardiomyocytes, and hepatocytes. Their ability to differentiate into various cell types makes them crucial for research in developmental biology and disease modeling, as well as for regenerative medicine applications. The CRISPR/Cas9 technology enables iPSCs to perform gene editing and repair functions. Continuous scientific progress ensures that Mouse iPSCs will increasingly be utilized in disease modeling as well as drug development and regenerative medicine applications.
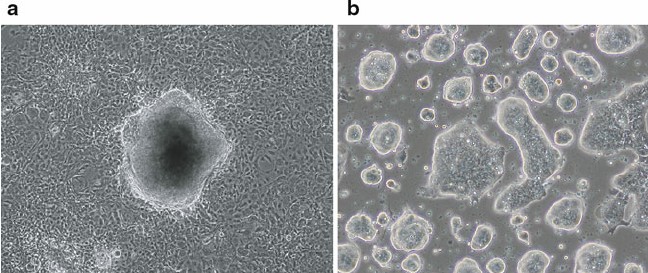 Fig. 1. Derivation of mouse fibroblast-derived iPSCs. (a) Morphology of mouse iPSC colony derived from fibroblasts (with three iPSC factors: Oct4, Sox2, Klf4) just before picking. (b) Expansion culture of fibroblast-derived mouse iPSCs (Imamura M, Okuno H, et al., 2012).
Fig. 1. Derivation of mouse fibroblast-derived iPSCs. (a) Morphology of mouse iPSC colony derived from fibroblasts (with three iPSC factors: Oct4, Sox2, Klf4) just before picking. (b) Expansion culture of fibroblast-derived mouse iPSCs (Imamura M, Okuno H, et al., 2012).
Shaking Culture Influenced the Size and Aggregation Profile of Chondrogenically Induced iPSC (CI-iPSC) Constructs
Induced pluripotent stem cells (iPSCs) offer unlimited self-renewal and pluripotency, making them promising for cartilage therapy. Despite their potential, there are limited protocols for efficient chondrogenic differentiation of iPSCs, especially involving mechanical stimuli. Limraksasin et al. investigated how shaking culture affects chondrogenic differentiation in mouse iPSCs based on the hypothesis that applying dynamic mechanical loading improves differentiation.
Mouse iPSCs were cultured in low-cell-attachment 96-well U bottom plates for 24 hours (Fig. 1A). They were then transferred to a chondrogenic induction medium. After 3 days, cell aggregates were moved to plates for suspension shaking culture using a see-saw shaker at 0 (static), 0.3, or 0.5 Hz. By day 21, CI-iPSC constructs in shaking culture grew significantly larger than in static culture (P < 0.01, Fig. 1B). The initial aggregates, 780 μm at day 1, increased to 1.1 mm by day 21 with shaking, while in static culture, they decreased to 660 μm (Fig. 1B). A live/dead assay showed mostly live cells in all groups. Histological staining was used to evaluate chondrogenic features in the constructs. Hematoxylin and eosin staining showed more cell clusters with ECM in shaking cultures than in static ones (Fig. 1D). Toluidine blue staining confirmed chondrogenesis enhancement in shaking cultures, showing more positive areas compared to static cultures (P < 0.01, Fig. 1E).
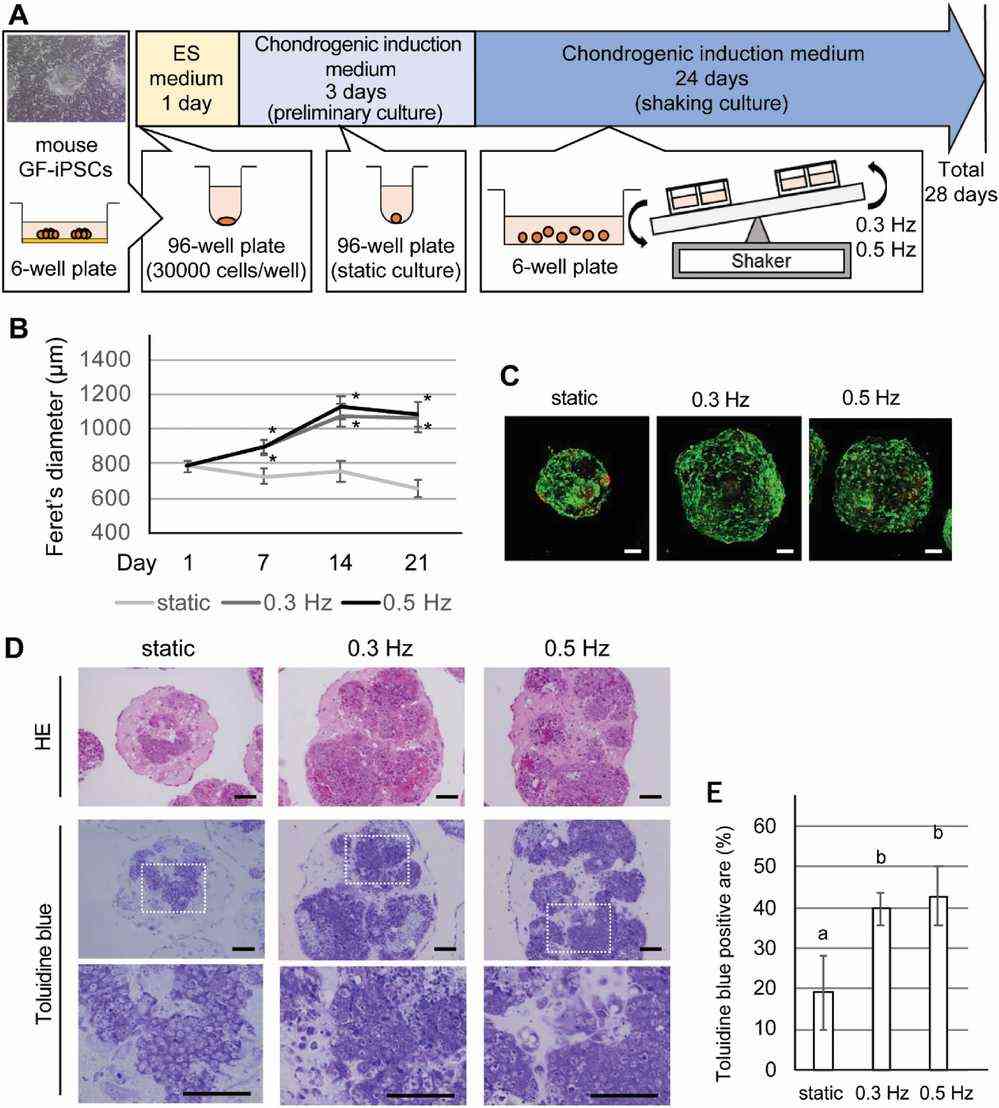 Fig. 1. Effects of a shaking culture on chondrogenically induced iPSC (CI-iPSC) construct aggregation profiles (Limraksasin P, Kosaka Y, et al., 2020).
Fig. 1. Effects of a shaking culture on chondrogenically induced iPSC (CI-iPSC) construct aggregation profiles (Limraksasin P, Kosaka Y, et al., 2020).
Generation of Functional DCs from Mouse iPSCs
Dendritic cells (DCs) are crucial in cancer immunotherapy, but obtaining sufficient functional DCs is challenging. iPSCs offer a potential solution, serving as an unlimited DC source. Oba et al. generated dendritic cells (DCs) from mouse embryonic fibroblasts-derived iPSC clones, 2A-4F-118 and 2A-4F-136. Mouse iPSCs were differentiated into DCs using a modified protocol incorporating notch signaling (Fig. 2A). First, iPSCs were grown on OP9 feeder layers for 6 days (step 1). On day 6, cells were moved to OP9-DL1 with GM-CSF for 7 days (step 2). By day 26, most iPSC-derived cells were CD11c and MHC class II positive, similar to bone marrow-derived DCs (Fig. 2B). They expressed CD24, CD11b, and DEC205, indicating conventional type 2 DC characteristics (Fig. 2C and D). Further experiments focused on clone 2A-4F-118.
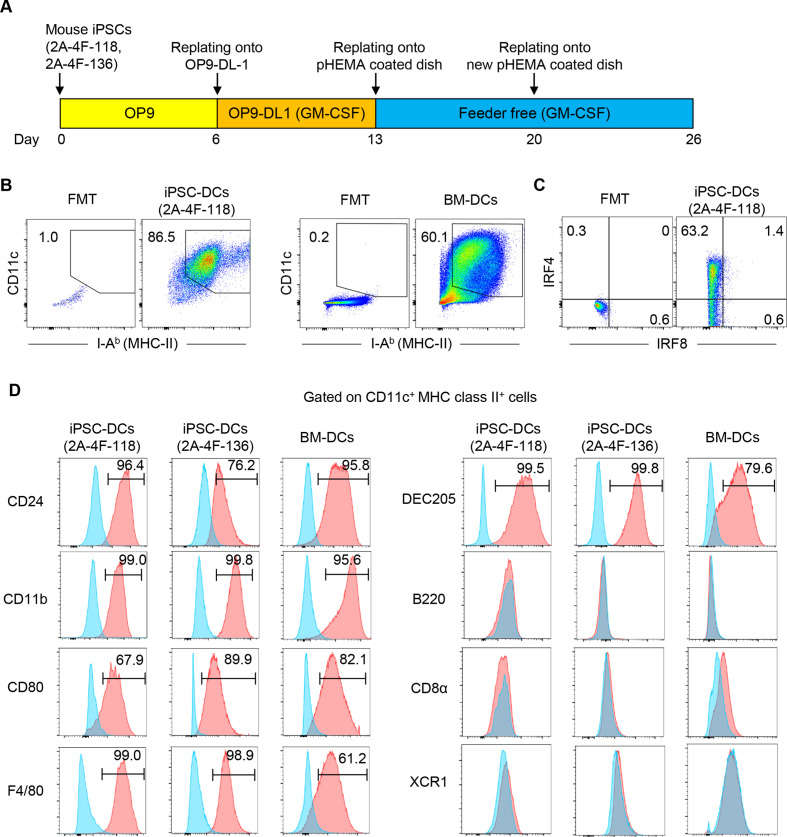 Fig. 2. Generation of dendritic cells (DCs) from mouse induced pluripotent stem cells (iPSCs) (Oba T, Makino K, et al., 2021).
Fig. 2. Generation of dendritic cells (DCs) from mouse induced pluripotent stem cells (iPSCs) (Oba T, Makino K, et al., 2021).
Ask a Question
Write your own review

