Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
LMSU
Cat.No.: CSC-C6416J
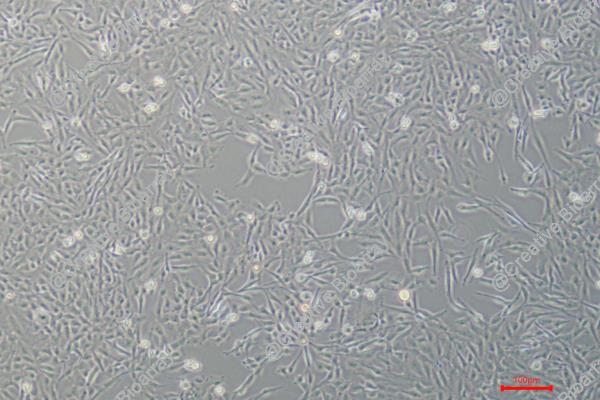
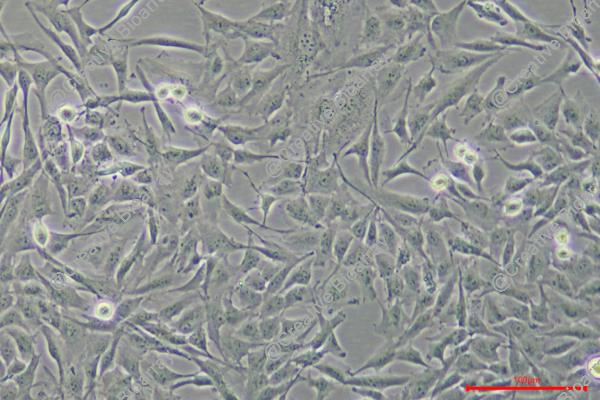
Species: Human
Morphology: epithelial-like
Culture Properties: Adherent cells
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Store in liquid nitrogen.
The LMSU cell line was established from a lymph node metastasis of a patient diagnosed with gastric cancer. Lymph nodes are common sites for metastatic spread in gastric cancer, reflecting the aggressive nature of the disease and its ability to disseminate through the lymphatic system. The cells from the metastatic lymph node were isolated, cultured, and characterized to develop a stable cell line that retains the genetic and phenotypic characteristics of the original tumor.
Therefore, the LMSU cell line is a valuable research tool for studying the biology of cancer metastasis. These cell lines can provide insights into the genetic and molecular changes that allow cancer cells to break away from the primary tumor, invade surrounding tissues, enter the lymphatic or circulatory system, and establish new tumors at distant sites in the body.
As a well-established and widely-used cell line, the LMSU model has contributed to advancing our knowledge of gastric cancer biology and the metastatic process. Continued research using this and other metastatic cancer cell lines remains crucial for developing more effective strategies for the diagnosis, treatment, and prevention of gastric and other types of metastatic cancer.
Evaluation of EGFR Signaling Effects in Gastric Cancer Cell Lines
Targeting epidermal growth factor receptor (EGFR) with the inhibitory antibody cetuximab may affect the motile behavior of tumor cells. The basal levels of gastric cancer cell motility were determined using time-lapse microscopy and quantification of the two-dimensional (2D) migration behavior based on two parameter values for each cell. Striking differences in the percentage of motile cells were observed between the various cell lines without treatment (Fig. 1): 96.26% (AGS), 88.84% (Hs746T), 68.29% (LMSU) and 49.81% (MKN1). These differences in the proportion of motile cells were reflected by a wide range of the mean average speed of untreated cells (Fig. 2): 45.00 μm/h (AGS), 39.73 μm/h (Hs746T), 35.44 μm/h (LMSU) and 13.88 μm/h (MKN1).
Comparisons of untreated and EGF-treated cells as well as EGF-treated and EGF/cetuximab-treated cells revealed no significant changes in the percentage of motile cells in the cell lines AGS, Hs746T and LMSU (Fig. 1). In MKN1 cells, EGF treatment increased the percentage of motile cells. Two cell lines reacted to EGF treatment with increased average speed compared to untreated cells: AGS and MKN1 cells (Fig. 2). Significant reversion of the EGF-induced increase in average speed was observed in the cell line AGS at 1 μg/ml cetuximab compared to EGF-treated cells. In MKN1 cells, a concentration-dependent decrease in the average speed due to cetuximab treatment was observed but was not statistically significant. No remarkable changes in average speed were observed in Hs746T and LMSU cells upon EGF and/or cetuximab treatment.
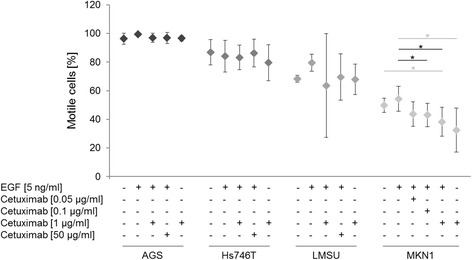 Fig. 1 Effect of EGF and/or cetuximab treatment on the percentage of motile cells. (Keller S, et al., 2017)
Fig. 1 Effect of EGF and/or cetuximab treatment on the percentage of motile cells. (Keller S, et al., 2017)
![Effect of EGF and/or cetuximab treatment on the average speed [μm/h]. For the determination of the descriptor “average speed”, cell numbers and conditions were identical to those in Fig. 1. P-values at significance levels of ≤ 0.050 are indicated by (*).](/upload/image/4-lmsu-2.jpg) Fig. 2 Effect of EGF and/or cetuximab treatment on the average speed [μm/h]. (Keller S, et al., 2017)
Fig. 2 Effect of EGF and/or cetuximab treatment on the average speed [μm/h]. (Keller S, et al., 2017)
Enforced Expression of Mutant P53 Promotes GEA Primary Tumorigenesis
Gastric and esophageal adenocarcinoma (GEA) is one of the main causes of cancer-related mortality worldwide. Functional studies showed that mutant p53 was sufficient, but not necessary, for enhancing primary tumor growth. 3 genetically modified isogenic versions of the LMSU (endogenous R175H) cell line were generated: a double control that expressed a nontargeting sgRNA/Cas9 and GFP (sgRNA con + GFP); a mutant p53-KO cell line that expressed p53 sgRNA#1 and GFP (p53 sg#1 + GFP); and a mutant p53 cell line that expressed p53 sgRNA#1 and a cDNA construct overexpressing p53-R175H' (p53 sg#1 + R175H'). The results showed deletion of endogenous mutant p53-R175H did not significantly alter adherent proliferation of luciferase-labeled LMSU gastric cancer cells (Fig. 3A). However, enforced expression of p53-R175H' in LMSU cells that lost endogenous mutant p53 via sgRNA#1 promoted proliferation by luciferase assays (Fig. 3A). A similar pattern was observed when these genetically modified cells were cultured in ultra-low attachment and soft agar conditions (Fig. 3B and C).
Xenograft experiments in nude mice showed that although endogenous mutant p53-R175H was nonessential for the growth of LMSU gastric cancer cells in vivo, overexpression of mutant p53-R175H promoted primary tumor growth (Fig. 3D). Transcript and protein levels of mutant p53 as determined by real-time PCR (RT-PCR) and IHC were maintained throughout the experiment as assessed at the endpoint (Fig. 3E). It is noteworthy that endogenous p53-R175H was highly expressed in LMSU cells while grown in xenograft primary tumors compared with adherent culture (Fig. 3E). Given these promising findings, whether enforced expression of hotspot p53 mutants can promote primary tumor properties in HGC27 was wondered, a gastric cancer cell line with an endogenous frameshift mutation in TP53 and therefore presumably defective for WT p53 activity. Unlike in LMSU cells, overexpression of hotspot p53 mutants (Fig. 3F) did not promote the proliferation of HGC27 cells during adherent culture. By contrast, enforced expression of p53-R175H and p53-R273H promoted ultra-low attachment and soft agar colony growth (Fig. 3G and H) to a greater extent than p53-R248Q in HGC27 cells. These data indicate that although endogenous mutant p53 is not required, overexpression can enhance primary tumor properties in GEA.
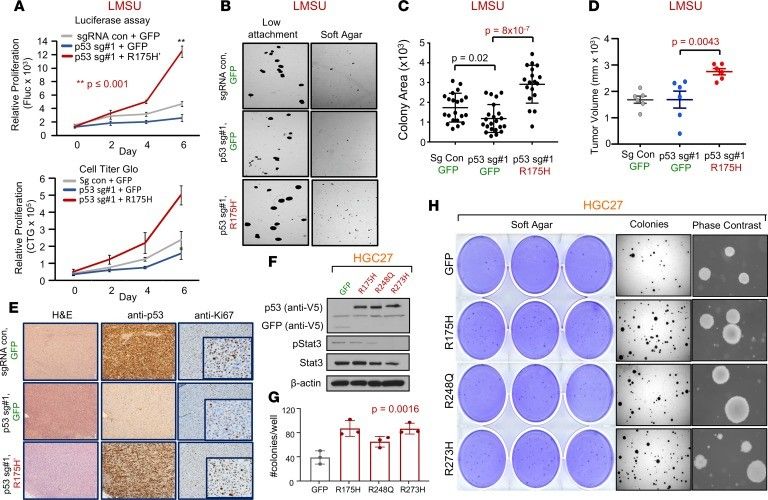 Fig. 3 Enforced expression of mutant p53 in 2 gastric adenocarcinoma cell lines confers a growth advantage. (Sethi N, et al., 2019)
Fig. 3 Enforced expression of mutant p53 in 2 gastric adenocarcinoma cell lines confers a growth advantage. (Sethi N, et al., 2019)
Tumor cells are more adaptable to the culture environment because they have autocrine production of growth-promoting substances.
The LMSU cell line was derived from a lymph node metastasis of a gastric (stomach) cancer.
While there are other gastric cancer cell lines available, the LMSU line is unique in that it was specifically derived from a lymph node metastasis. This makes it a valuable model for studying the specific genetic and molecular changes associated with the metastatic process in gastric cancer.
Researchers have utilized the LMSU cell line to investigate a variety of topics related to gastric cancer progression and metastasis, such as cell signaling pathways, tumor angiogenesis, and the identification of potential therapeutic targets.
Ask a Question
Average Rating: 4.7 | 3 Scientist has reviewed this product
Long-term cooperation
Due to the high quality and good service of the Creative Bioarray's products, our laboratory has established a long-term relationship with them.
19 Apr 2022
Ease of use
After sales services
Value for money
An invaluable tool
As a researcher focused on understanding the mechanisms of cancer metastasis, the LMSU cell line has been an invaluable tool in my work.
15 Mar 2024
Ease of use
After sales services
Value for money
Reliable products
I've purchased the cells multiple times from Creative Bioarray and have always received a reliable, well-characterized product.
08 Jan 2024
Ease of use
After sales services
Value for money
Write your own review
- You May Also Need