Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
Immortalized Mouse Lung Epithelial Cells (MLE-15)
Cat.No.: CSC-I9200L
Species: Mouse
Source: Distal respiratory epithelium
Morphology: Polygonal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Publications
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
The MLE-15 cell line develops from the respiratory epithelium of a 5-month-old FVB/N mouse. Scientists made the MLE-15 cells immortal by adding SV40 (Simian virus 40) to them. The mouse lung contains pulmonary epithelial cells that line the inner surfaces of airways and alveoli as a barrier to both gas exchange and external pathogens. Under a microscope MLE-15 cells show normal epithelial cell features which appear as flat polygonal structures with clear cell borders and strong intercellular connections that create a dense cell layer. They express high levels of various pulmonary epithelial cell markers, such as members of the cytokeratin family like CK8 and CK18.
The MLE-15 cell line helps scientists better understand respiratory diseases like ARDS, cystic fibrosis, emphysema, and pneumonia through laboratory experiments. Scientists use these cells to test how lung epithelial cells react to external influences while developing new medical treatments.
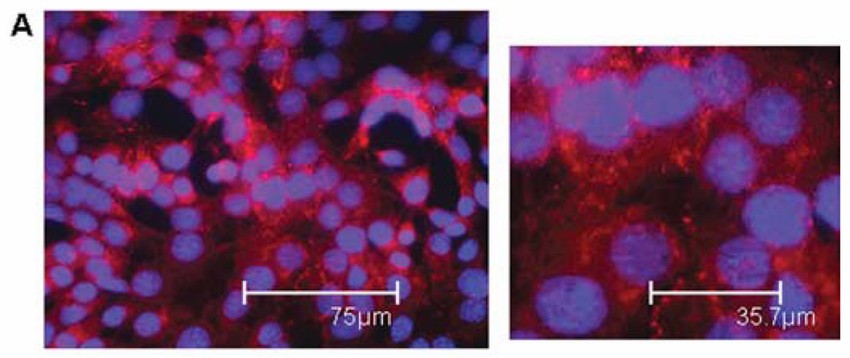 Fig. 1. MLE-15 cells grown in standard cell culture conditions. Stains: ToPro-3 nuclear stain (blue) and anti-zona occludins (ZO-1) antibody (red) (Grek CL, Newton DA, et al., 2009).
Fig. 1. MLE-15 cells grown in standard cell culture conditions. Stains: ToPro-3 nuclear stain (blue) and anti-zona occludins (ZO-1) antibody (red) (Grek CL, Newton DA, et al., 2009).
MicroRNA-598 Inhibition Ameliorates LPS-Induced Acute Lung Injury in Mice through Upregulating Ebf1 Expression
Acute lung injury is a severe form of acute respiratory distress syndrome (ARDS) characterized by high morbidity and mortality rates. MicroRNAs (miRNAs) have emerged as crucial regulators in the development of acute lung injury. MiR-598 has been identified to inhibit cancer cell behaviors including proliferation, migration, invasion. Nevertheless, its role in acute lung injury, especially concerning the disruption of epithelial and endothelial barriers, remains unclear.
Zhao et al. found MiR-598 expression is upregulated in lung tissues of mice with LPS-induced acute lung injury. And inhibition of miR-598 attenuated inflammatory response, oxidative stress, and lung injury in mice treated with LPS, while overexpression of miR-598 exacerbated the LPS-induced acute lung injury. Bioinformatics analysis showed Ebf1 is a downstream target of miR-598. They further explored the role of Ebf1 in LPS-induced acute lung injury by overexpressing it in MLE-15 cells (Fig. 1a and b). LPS increased TNF-α and IL-6 levels, but Ebf1 overexpression reduced these cytokines (Fig. 1c). Additionally, LPS caused oxidative stress by boosting ROS production, which Ebf1 overexpression alleviated (Fig. 1d). LPS also inhibited cell proliferation and triggered apoptosis, effects that were partially reversed by Ebf1 overexpression (Fig. 1e–g). To examine the interaction between miR-598 and Ebf1, they knocked down Ebf1 in MLE-15 cells using siRNA (Fig. 2a). While miR-598 inhibition reduced LPS-induced TNF-α and IL-2, Ebf1 knockdown increased these cytokines and countered miR-598's effects (Fig. 2b). Similarly, LPS-induced ROS production, reduced cell proliferation, and increased apoptosis were mitigated by miR-598 inhibition but further exacerbated by Ebf1 knockdown (Fig. 2c–g). The protective effects of miR-598 inhibition on oxidative stress, proliferation, and apoptosis were nullified by Ebf1 knockdown (Fig. 2c–g).
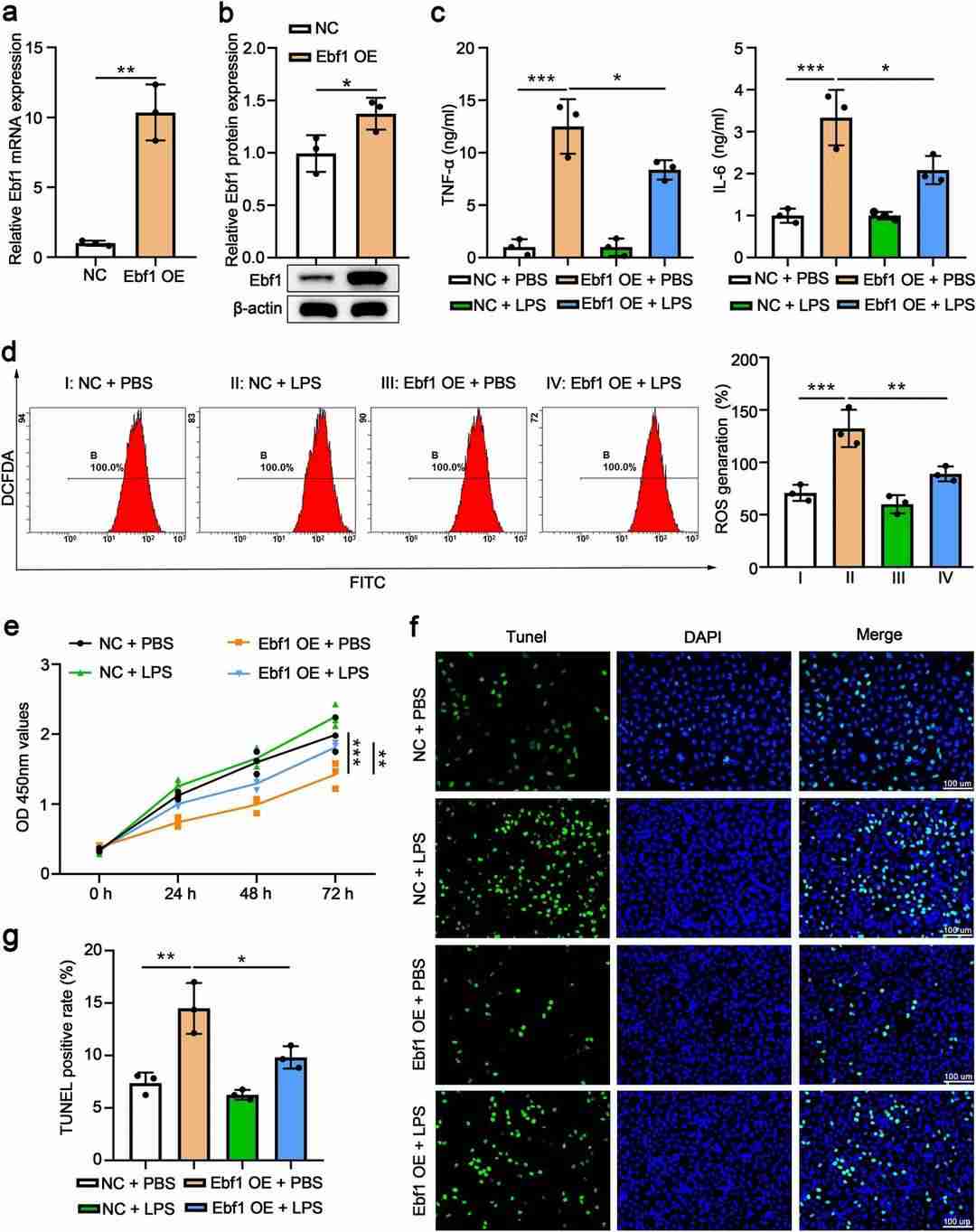 Fig. 1. Ebf1 overexpression attenuates LPS-induced inflammation, oxidative stress, impaired proliferation, and apoptosis in MLE-15 (Zhao Q, He L, et al., 2023).
Fig. 1. Ebf1 overexpression attenuates LPS-induced inflammation, oxidative stress, impaired proliferation, and apoptosis in MLE-15 (Zhao Q, He L, et al., 2023).
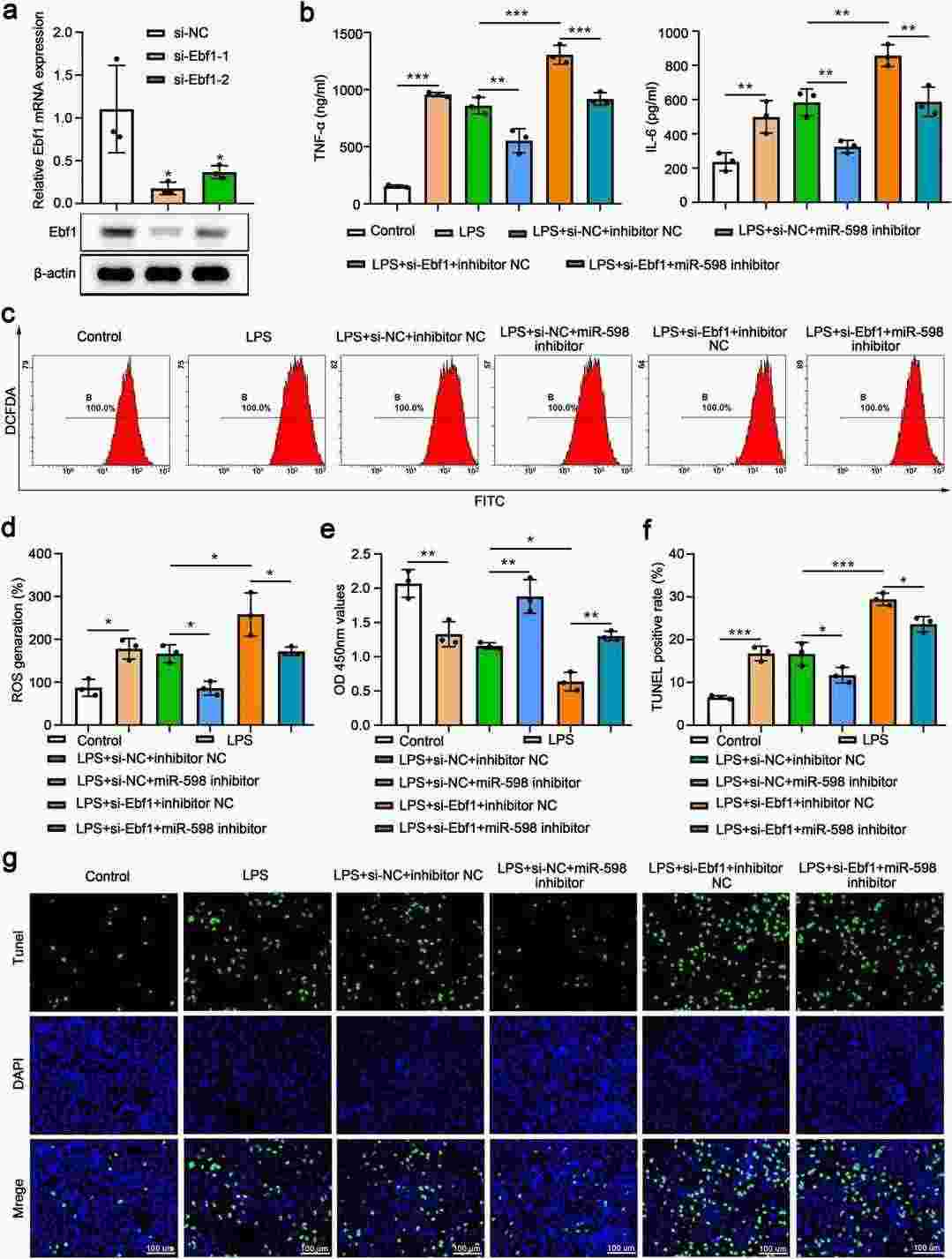 Fig. 2. The protective effects of miR-598 inhibition in MLE-15 cells were abolished by Ebf1 knockdown (Zhao Q, He L, et al., 2023).
Fig. 2. The protective effects of miR-598 inhibition in MLE-15 cells were abolished by Ebf1 knockdown (Zhao Q, He L, et al., 2023).
Generation of MLE-15/HPS Clones and Validation of Gene Expression
Hermansky-Pudlak syndrome (HPS) encompasses genetic disorders resulting from gene defects in protein and membrane targeting complexes related to lysosome-related organelles, notably affecting lung tissue and causing significant morbidity via pulmonary fibrosis. Despite using mouse models, understanding of HPS-related alveolar type 2 (AT2) cell injury remains limited due to insufficient cellular models.
Kook's team aims to overcome these challenges by leveraging CRISPR/Cas9 gene editing to generate HPS-specific mutations in MLE-15 cell lines, targeting genes responsible for HPS-related fibrotic lung disease. Using two guide RNAs, they targeted larger genome segments for editing by creating two Cas9-induced double-strand breaks, leading to deletions visible through gel electrophoresis of genomic PCR products. Figure 3 illustrates this: Figure 3D shows a single PCR product from a large deletion on both alleles in the MLE-15/HPS9 clone. Typically, most clones displayed the anticipated large deletion on one allele and a small indel on the other, as seen in Figure 3B for MLE-15/HPS2 and Figure 3C for MLE-15/HPS3. Figure 3A shows the MLE-15/HPS1 clone with a small indel on one allele and a large nonspecific sequence insertion on the other. Sequencing genomic PCR products distinguished small indels from WT sequences, eliminating 0.1% heterozygous clones. We also transfected MLE-15 cells with an empty pX459 vector to assess the impact of puromycin selection, which showed no significant effects compared to WT cells (labeled pX459 in Figure 3).
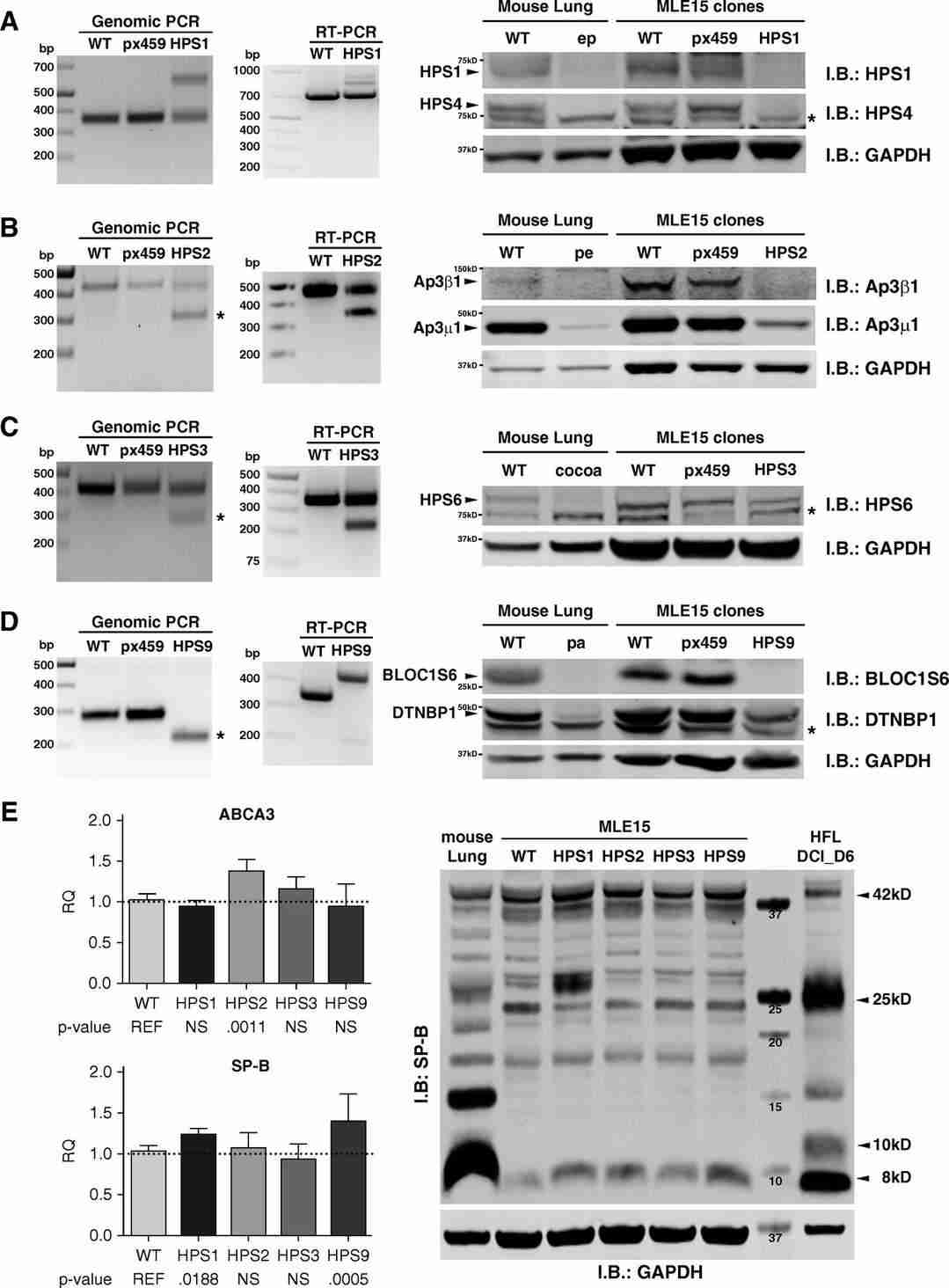 Fig. 3. Confirmation of Hermansky-Pudlak syndrome (HPS) gene editing in MLE-15 cells (Kook S, Qi A, et al., 2018).
Fig. 3. Confirmation of Hermansky-Pudlak syndrome (HPS) gene editing in MLE-15 cells (Kook S, Qi A, et al., 2018).
MLE-15 cells are highly versatile and can be utilized in numerous research areas, including:
Investigating lung development and differentiation processes.
Studying the pathophysiology of respiratory diseases, such as asthma and chronic obstructive pulmonary disease (COPD).
Researching the effects of environmental pollutants on lung cells.
Evaluating drug responses and therapeutic interventions in pulmonary medicine.
Exploring epithelial barrier function and homeostasis in lung tissue.
We provide a comprehensive culture protocol with each order that includes:
Recommended culture media and supplements necessary for optimal growth.
Guidelines for seeding densities and passaging techniques.
Required environmental conditions, including temperature, humidity, and CO2 levels.
Ask a Question
Write your own review

