Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
Immortalized Mouse Kupffer Cells (ImKC)
Cat.No.: CSC-I9264L
Species: Mus musculus
Source: Liver
Morphology: Macrophage-like
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
2) Western blot analysis on p53 expression;
3) qRT-PCR used to analyze level of LPS induced cytokine production.
The Immortalized Mouse Kupffer Cells (ImKC) cell line develops from H-2Kb-tsA58 transgenic male mice which express the thermally stable tsA58 mutant of the SV40 large T antigen. The identification of these cells relies on the F4/80 marker that exclusively labels Kupffer cells. Compared to primary cells, ImKC cells exhibit immortality, high levels of p53 expression, and increased telomerase activity. Additionally, the ImKC cell line can proliferate at 37°C and produce pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β under lipopolysaccharide (LPS) induction. These characteristics make the ImKC cell line an important tool for studying ImKC cells serve as a model to study the development of liver diseases including inflammation and injury of the liver along with non-alcoholic fatty liver disease.
Researchers can utilize the ImKC cell line to study how liver diseases such as liver inflammation, liver injury, and non-alcoholic fatty liver disease develop. Research on Kupffer cell involvement in liver disease development is possible through analysis of ImKC cell secretions and functional changes under various stimulatory environments. The immortal status of the ImKC cell line makes it a renewable cell source for consistent long-term experimental use and high-throughput screenings.
ZC Inhibits LPS-Induced ROS Production in Immortalized Mouse Kupffer Cells (ImKCs)
Non-alcoholic fatty liver disease (NAFLD) sees no FDA-approved treatments despite its significant prevalence. Key factors in NAFLD progression include Kupffer cell activation and mtROS, driving inflammation and liver damage. This study investigates Zaluzanin C (ZC), a compound from Laurus nobilis, for its potential in modulating mtROS to alleviate inflammation and hepatic steatosis.
Using Immortalized Mouse Kupffer Cells (ImKCs) and Hepatocytes, Jung et al. examines ZC's effects on mtROS production, inflammatory cytokine expression, mitochondrial function, lipogenesis, and gene expression related to fatty acid oxidation and mitochondrial biosynthesis, thereby assessing ZC's hepatoprotective properties. LPS binds to TLR-4, inducing mtROS generation. To find the optimal ZC concentration, they tested its cytotoxicity in ImKCs from 5 μM to 1000 μM (Fig. 1B). ZC showed no toxicity or growth inhibition up to 10 μM and effectively reduced LPS-induced mtROS (Fig. 1C and D), similar to the mtROS inhibitor Mito-TEMPO. ZC also mitigated LPS-induced SOD1 protein decline, suggesting its antioxidant role in preserving SOD1 in ImKCs. TNF-α induces mtROS in hepatocytes during inflammation. Testing ZC's cytotoxicity in Hep3B and primary hepatocytes showed no toxicity up to 10 μM (Fig. 2A). ZC reduced mtROS in hepatocytes, resembling Mito-TEMPO's effect (Fig. 2E and F). TNFR1 knockdown in primary hepatocytes decreased mtROS, indicating TNF-α's role via TNFR1 (Fig. 2D). ZC inhibits mtROS in Hep3B by modulating Kupffer cell activity and reducing TNF-α-induced mtROS.
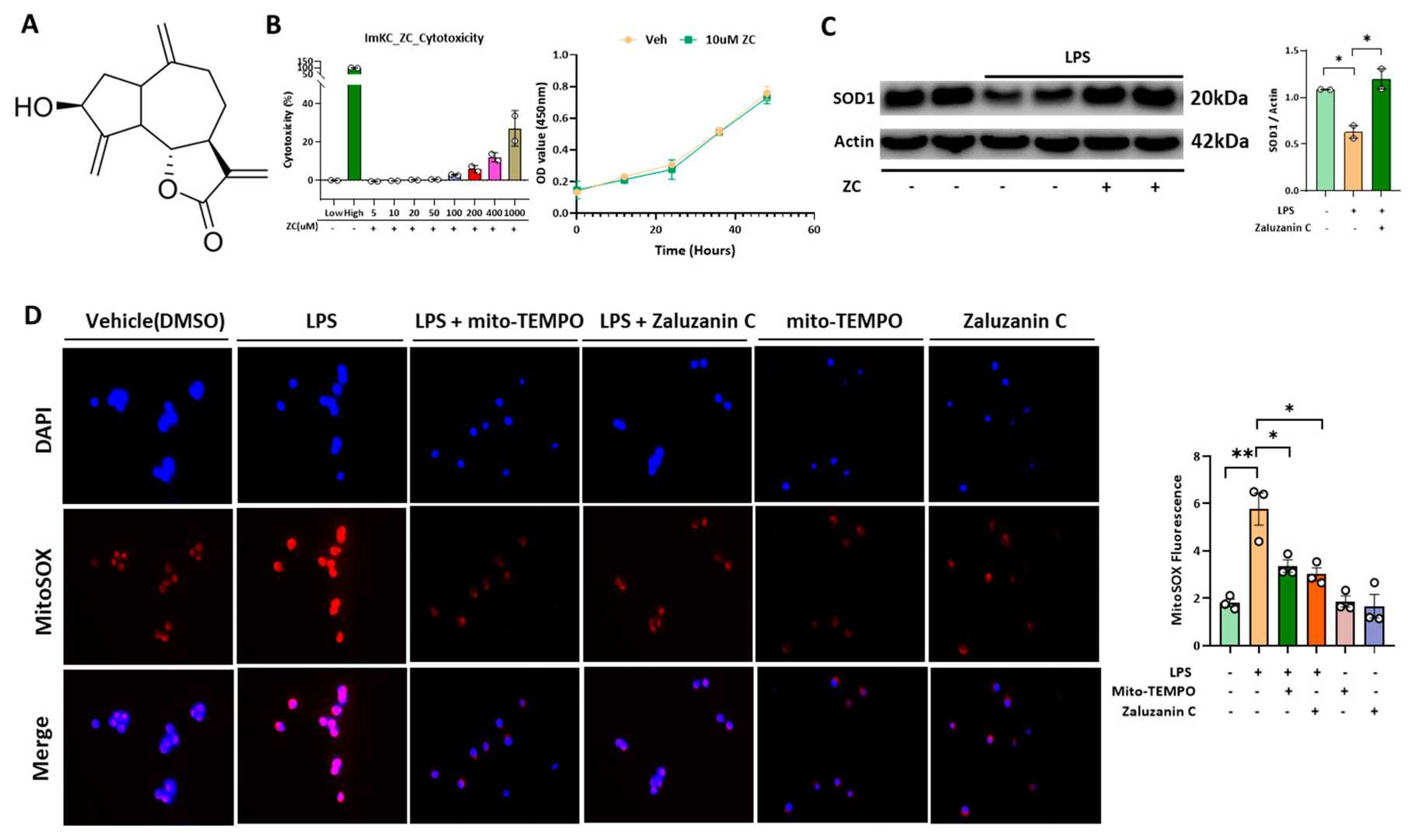 Fig. 1. ZC inhibits LPS-induced ROS in Immortalized mouse kupffer cells (ImKC) (Jung J, Wang F, et al., 2023).
Fig. 1. ZC inhibits LPS-induced ROS in Immortalized mouse kupffer cells (ImKC) (Jung J, Wang F, et al., 2023).
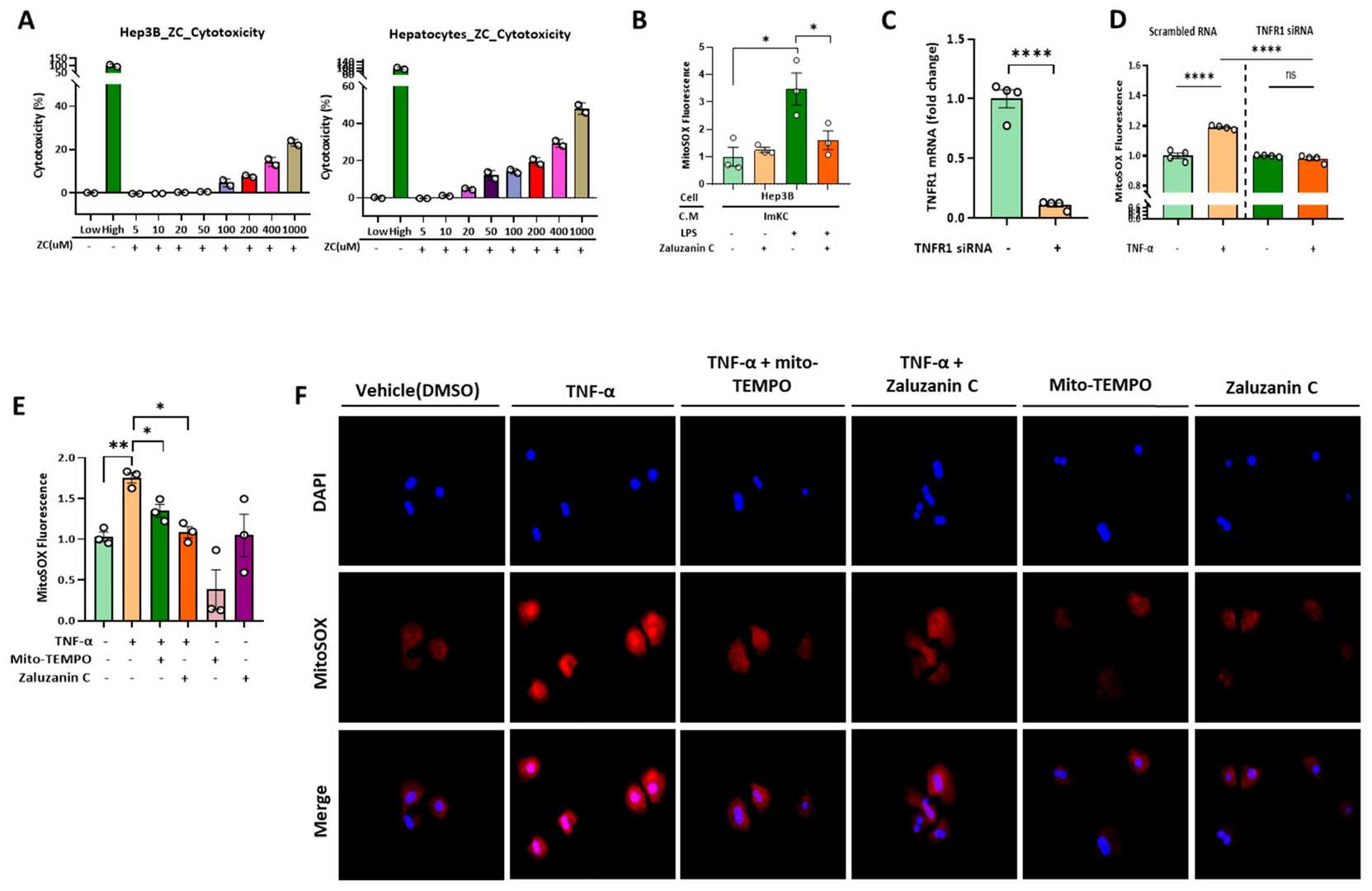 Fig. 2. ZC inhibits ROS in hepatocytes induced by TNF-α secreted by Immortalized mouse kupffer cells (ImKCs) (Jung J, Wang F, et al., 2023).
Fig. 2. ZC inhibits ROS in hepatocytes induced by TNF-α secreted by Immortalized mouse kupffer cells (ImKCs) (Jung J, Wang F, et al., 2023).
LPS Diminishes USP13 Levels, but not USP13 mRNA, in Kupffer Cells
Protein ubiquitination controls protein stability and cellular functions, with deubiquitinases like USP13 reversing this process. Despite its role in inflammation-related cellular responses USP13 lacks clear understanding of its molecular regulation mechanisms. Researchers examine the degradation process of USP13 in Kupffer cells (ImKC immortalized mouse Kupffer cells) after exposure to lipopolysaccharide (LPS).
Kupffer cells, liver macrophages, are crucial in liver inflammation and repair. Fan's team investigated how LPS affects USP13 levels in these cells. LPS, starting at 10 ng/ml, decreased USP13 levels after 24 hours (Figure 2a–d), similar to results in Raw264.7 cells. Serum concentration changes did not affect this reduction, indicating that USP13 levels drop in Kupffer cells, not hepatocytes. This may result from different TLR4 and cytokine receptor expression levels between the cells. LPS treatment decreased USP13 mRNA in Raw264.7 cells without affecting the mRNA levels in Kupffer cells as shown in Figure 3a. The Western blot results revealed that LPS decreased USP13 protein levels without changing its mRNA levels as shown in Figure 3b indicating LPS influences USP13 protein stability.
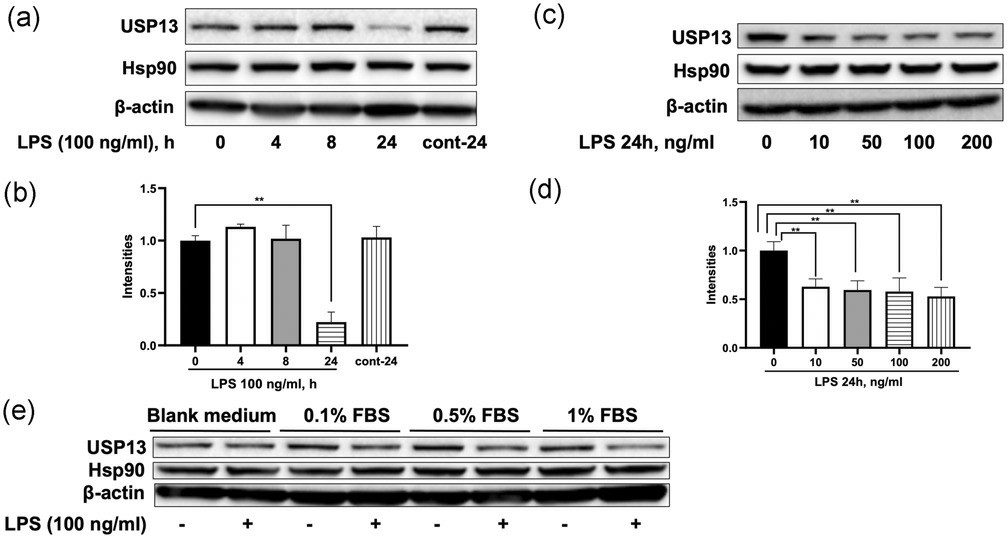 Fig. 3. LPS reduces USP13 levels in Kupffer cells (Fan Y, Li Y, et al., 2020).
Fig. 3. LPS reduces USP13 levels in Kupffer cells (Fan Y, Li Y, et al., 2020).
Ask a Question
Write your own review


