Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
Immortalized Mouse Astrocytes-SV40T (IMA2.1)
Cat.No.: CSC-I9175L
Species: Mus musculus
Source: Cerebral Cortex
Morphology: star-shape morphology
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
CSC-C4465X Mouse Astrocytes Cortex
CSC-C4466X Mouse Astrocytes Cerebellar
CIK-HT003 HT® Lenti-SV40T Immortalization Kit
1) immunostaining to assess NF-kB translocation from the cytosol to the nucleus;
2) RT-qPCR and quantitative PCR detection of NOS-2 mRNA levels;
3) Western Blot analysis of NOS-2 and COX2 proteins.
The Immortalized Mouse Astrocytes-SV40T (IMA2.1) cell line comes from mouse astrocytes modified with the SV40 Large T Antigen. Researchers often use these cells to study what astrocytes do and how they contribute to brain diseases. IMA 2.1 cells carry markers of immature astrocyte, like nestin and CD44. They can convert MPTP into MPP+ and change glutamate into glutamine. These cells are also very responsive to cytokines that cause inflammation, such as IL-1β, TNF-α, and IFN-γ. When exposed to these cytokines, they increase the activity of certain genes, like NOS-2 and COX-2, and release substances that cause inflammation.
Their sensitivity to inflammation makes IMA 2.1 cells perfect for lab models that explore how inflammation works. Scientists expose these cells to substances like lipopolysaccharide and various inflammatory cytokines such as TNF-α, IL-1β, and IFN-γ, either alone or in combination, to see how astrocytes react. This includes observing cellular morphological changes, the secretion of inflammatory factors, and the activation of intracellular signaling pathways under inflammatory stimuli. Because astrocytes are critical components of the nervous system, IMA 2.1 cells are also used to study changes during brain diseases and injuries, helping us understand how these conditions develop at the cellular level.
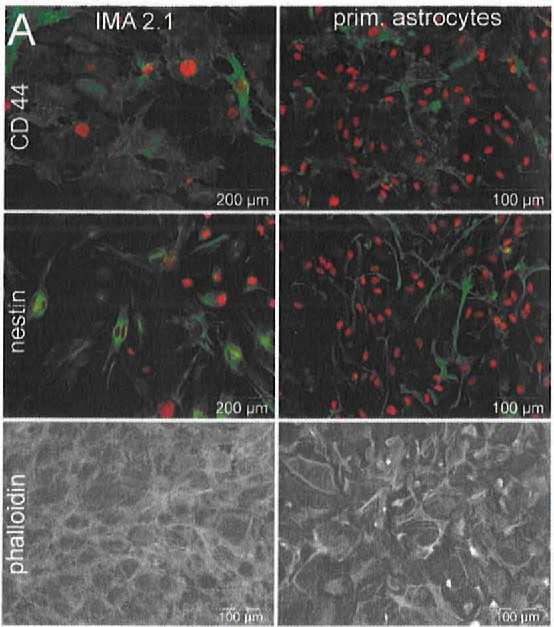 Fig. 1. Phenotypic and functional characteristics of IMA 2.1 and primary mouse astrocytes. They were stained for CD44 and nestin (in green) and their nuclei were marked with H-33342 (in red). Phalloidin was used to highlight cell shape by targeting F-actin (Schildknecht S, Kirner S, et al., 2012).
Fig. 1. Phenotypic and functional characteristics of IMA 2.1 and primary mouse astrocytes. They were stained for CD44 and nestin (in green) and their nuclei were marked with H-33342 (in red). Phalloidin was used to highlight cell shape by targeting F-actin (Schildknecht S, Kirner S, et al., 2012).
GPNMB Signaling in Astrocyte Cultures
Neuroinflammation, a pivotal feature of neurodegenerative diseases like Parkinson's disease (PD), involves glial cell activation and inflammatory response. Glycoprotein non-metastatic melanoma protein B (GPNMB), a transmembrane glycoprotein, has shown neuroprotective properties in diseases like ALS, yet its mechanism in PD remains underexplored. Using immortalized mouse astrocytes (IMA2.1) and primary mouse astrocytes (PMAs), Neal's team assesed GPNMB's impact on cytokine-induced astrocyte activation.
Following MPTP treatment, increased CD44 levels in astrocytes were observed. Then, they used the IMA2.1 and PMAs to assess GPNMB and CD44 expression. Previously, Yu et al. found IL-4 significantly increased GPNMB expression in mesenchymal stem cells, unlike LPS and IFNγ. Neal et al. tested if astrocytes behaved similarly by treating them with either pro-inflammatory cytokines (TNFα, IL-1β, IFNγ) or IL-4. Both cell types expressed GPNMB, with IL-4 enhancing expression, while pro-inflammatory cytokines did not (Fig. 1a and b). GPNMB protein rise was confirmed in astrocytes post-IL-4. Both cell types also expressed CD44, which increased with pro-inflammatory cytokines (Fig. 1c and d). IL-4 boosted CD44 protein but not gene expression. Increased CD44 may stem from reduced degradation. These findings suggest GPNMB-CD44 signaling adjusts inflammatory responses, with CD44 rising after pro-inflammatory stimuli and GPNMB after anti-inflammatory stimuli.
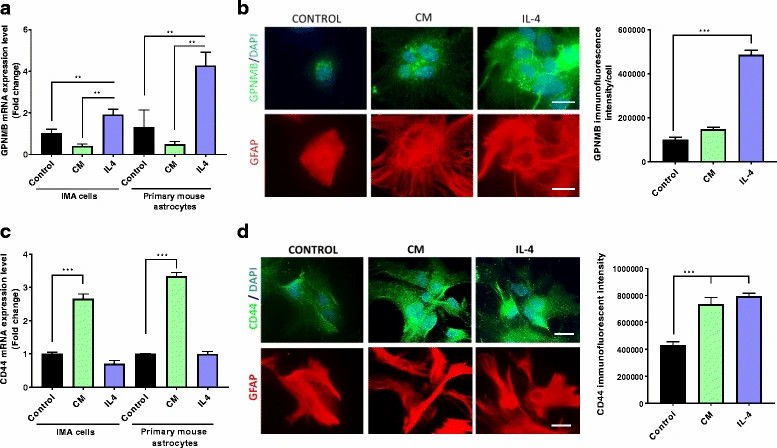 Fig. 1. Increased GPNMB and CD44 in cultured astrocytes exposed to different stimuli (Neal M L, Boyle A M, et al., 2018).
Fig. 1. Increased GPNMB and CD44 in cultured astrocytes exposed to different stimuli (Neal M L, Boyle A M, et al., 2018).
Establishment of the LUHMES and IMA Cells Co-culture Model
PD's development involves dopaminergic neuron degeneration, necessitating new human-based models for neuroprotective drug testing. Current models lack neuron-glia interactions vital for metabolism and neurotoxicity studies. Efremova's team developed an in vitro model using human dopaminergic neurons (LUHMES) co-cultured with Immortalized mouse astrocyte cell line (IMA 2.1), recreating MPTP metabolism.
For co-culturing with LUHMES neurons, IMA 2.1 or mixed primary glial cells were seeded in multi-well plates in DMEM with supplements at a density of 15,000 cm−2. After 24 hours, LUHMES differentiation medium was added for 2 days. Pre-differentiated LUHMES cells were then placed on astrocytes at 300,000 cells per well and differentiated for another 4 days. On day 6, the astrocyte:neuron ratio was 1.3, used for MPTP and drug treatments (Fig. 2B). Immunostaining with specific antibodies identified cell types, showing LUHMES layered on astrocytes. The culture maintained a reproducible neuron differentiation with IMA 2.1 astrocytes, similar to mono-cultures (Fig. 2C). Co-cultured neurons had more tyrosine hydroxylase and dopamine (Fig. 2D; Fig.3A) and expressed TH, DAT, and the vesicular monoamine transporter (Fig. 2E; Fig.3B).
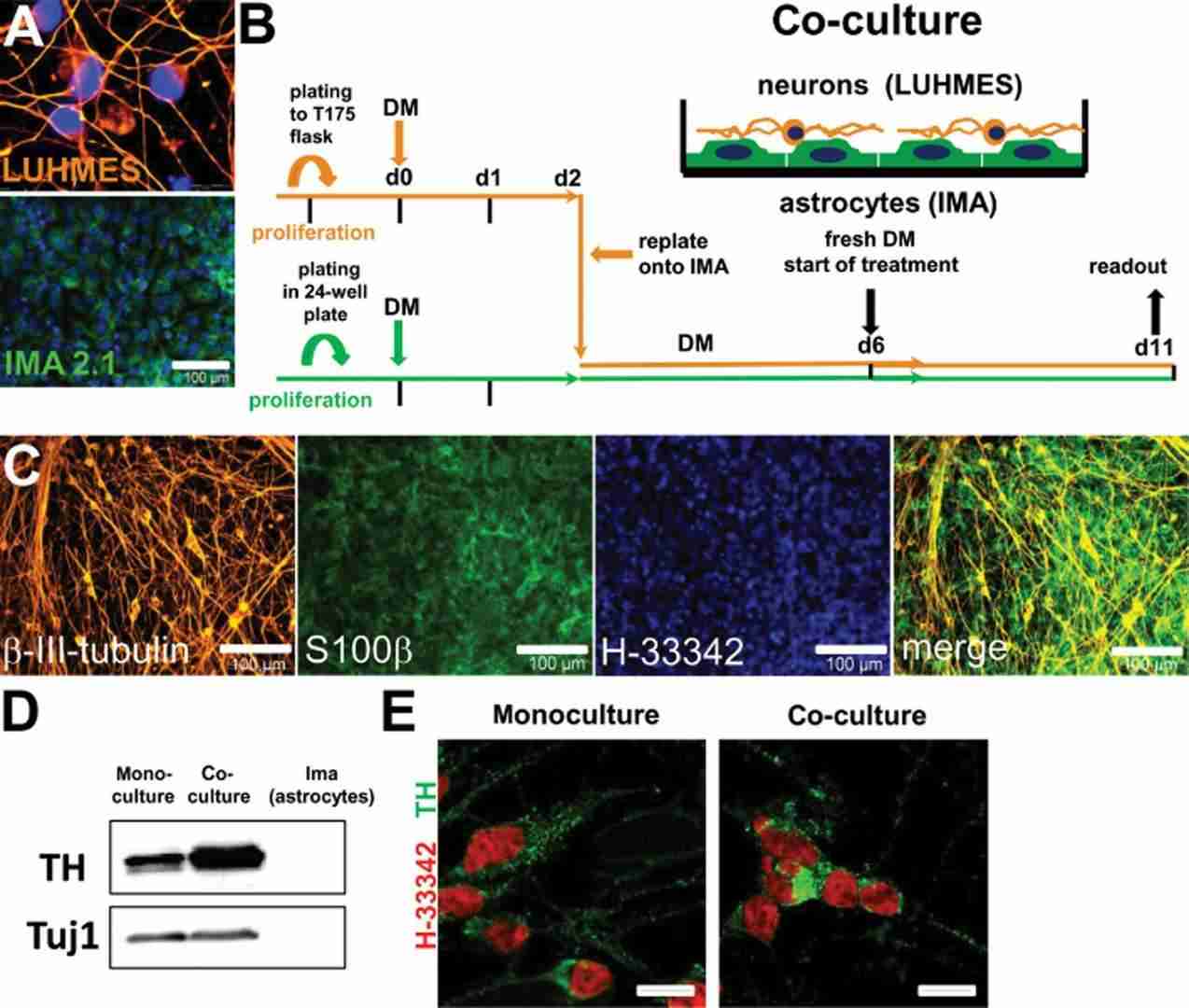 Fig. 2. Schematic representation and phenotypic characterization of neuron–astrocyte co-cultures (Efremova L, Schildknecht S, et al., 2015).
Fig. 2. Schematic representation and phenotypic characterization of neuron–astrocyte co-cultures (Efremova L, Schildknecht S, et al., 2015).
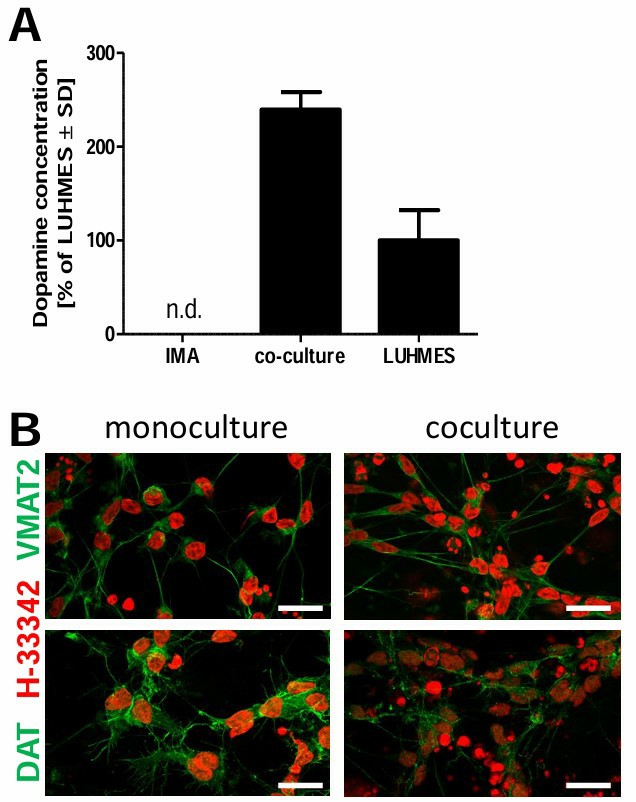 Fig. 3. Dopaminergic characteristics of LUHMES differentiated in mono-culture and in co-culture with astrocytes (Efremova L, Schildknecht S, et al., 2015).
Fig. 3. Dopaminergic characteristics of LUHMES differentiated in mono-culture and in co-culture with astrocytes (Efremova L, Schildknecht S, et al., 2015).
Ask a Question
Write your own review

