Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
BA/F3
Cat.No.: CSC-C2045
Species: Mouse
Source: pro B cells
Morphology: round (some polymorph) cells in suspension
Culture Properties: suspension
- Specification
- Background
- Scientific Data
- Publications
- Q & A
- Customer Review
b. Cell-Based assay
c. In vivo efficacy study
Immunology: CD11b (+), F4/80 (+)
Viruses: PCR: SMRV -
Store in liquid nitrogen.
Biosafety classification is based on U.S. Public Health Service Guidelines, it is the responsibility of the customer to ensure that their facilities comply with biosafety regulations for their own country.
Ba/F3 is a hematopoietic cell line derived from mouse bone marrow that depends on interleukin-3 (IL-3). Due to their low-level expression of the B-cell specific B220 antigen and the germline configuration of their immunoglobulin loci, Ba/F3 cells are classified as early cells of the lymphoblastic lineage. In the presence of IL-3, BA/F3 cells can proliferate and divide rapidly; however, upon removal of IL-3, the cells quickly lose viability and die.
BA/F3 cells are easily transfected and infected via electroporation or common retroviral and lentiviral expression systems. More than 700 engineered BA/F3 cell lines have been developed, covering most kinase families. For example, by introducing specific kinase mutation genes (such as KRAS, BRAF, etc.), cell lines that rely on mutation-driven genes rather than IL-3 can be constructed, which are useful for screening kinase inhibitors and studying drug resistance. Moreover, BA/F3 cells can be used for resistance studies; for instance, by using N-ethyl-N-nitrosourea (ENU) mutagenesis, resistant mutant-carrying cell lines can be generated. Additionally, BA/F3 cells can form tumor-like structures in mice, which can be used to evaluate the in vivo antitumor efficacy of kinase inhibitors.
Exogenous Janus Kinase 617 Codon Influences Small Noncoding RNAs and Gene Expression in Ba/F3 Cells
MPNs arise in bone marrow, potentially progressing to leukemia, with the JAK2V617F mutation highly present in various MPNs and within the general population, albeit with varying penetrance. Chen's team investigated JAK2V617F's role using next-generation sequencing of small and total RNAs in JAK2 versus JAK2V617F-expressing Ba/F3 cells.
Small RNAs were extracted and sequenced from Ba/F3 cells transfected with mock, vehicle, wild type JAK2, or V617F mutant JAK2 to check if exogenous JAK2 expression alters miRNA profiles. The annotated sequencing data showed distinct RNA expressions for different genetic backgrounds (Fig. 1a). Common miRNAs between humans and mice, excluding the Ba/F3 background, appear in Supplementary Table 2, with a heatmap of their clustering shown in Figure 1b. Using DAVID, we found these miRNAs are involved in various cancers. Potential targets like DNMT1, 3A/3B, Bmi1, UBC9, Ezh2, and Sirt1, indicate JAK2V617F might disrupt the epigenome. Altered miRNA expressions from JAK2V617F MPN patients' microarray data were compared, highlighting let7 as the common miRNA family shared across humans and mice, as confirmed in prior studies. RNA sequencing was performed to investigate global changes in transcripts and gene expression in Ba/F3 cells transfected with vehicle, wild type, or V617F mutant JAK2. Genes and transcripts specific to JAK2 and JAK2V617F, excluding the Ba/F3 background, were identified (Fig. 2a and b). In JAK2 and JAK2V617F-expressing cells, 1266 genes showed significant differential expression compared to the vehicle control. A heatmap in Figure 2c illustrates these differences among control, JAK2, and JAK2V617F Ba/F3 cells. Additionally, the expressional variations were cross-examined, and the differentially expressed genes were summarized in a Venn diagram (Fig. 2d).
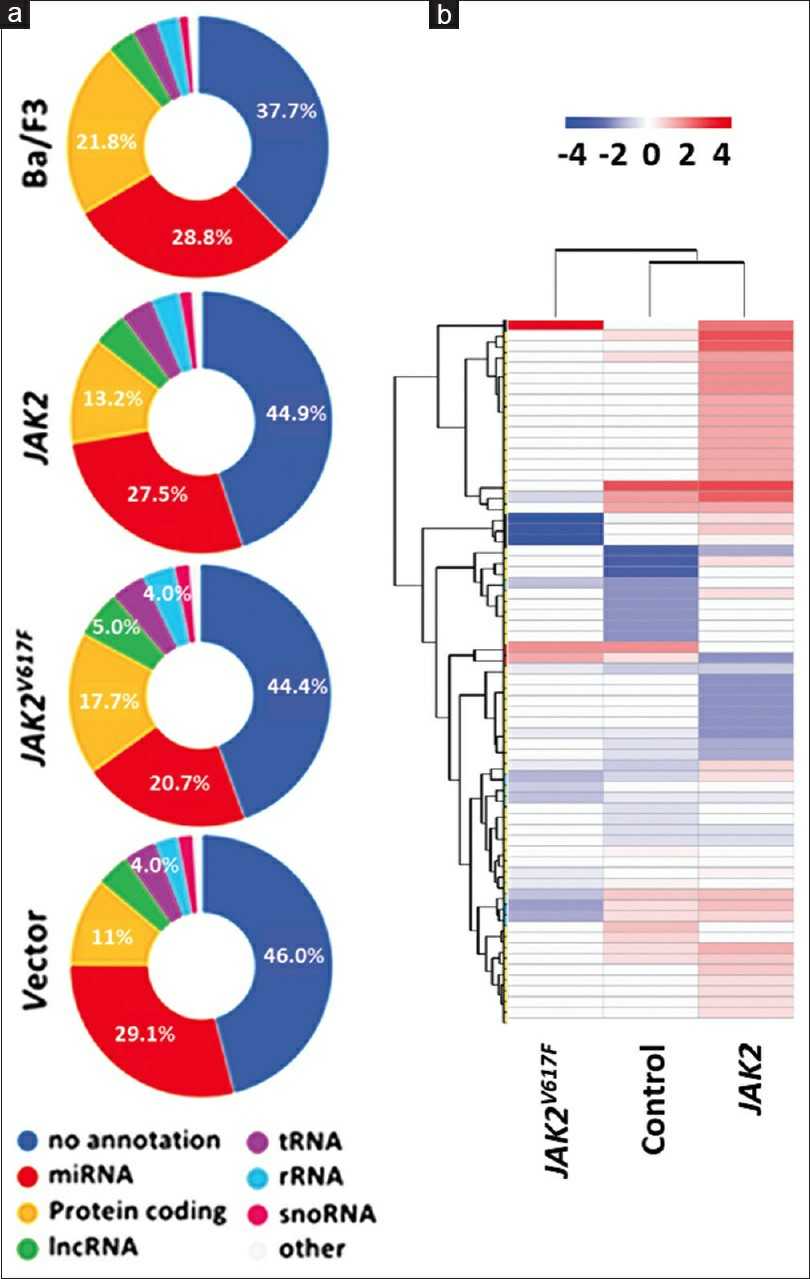 Fig. 1. Janus kinase 617 codon (JAK2V617F) mutation influences noncoding RNA expression (Chen Y, Wan Y, et al., 2024).
Fig. 1. Janus kinase 617 codon (JAK2V617F) mutation influences noncoding RNA expression (Chen Y, Wan Y, et al., 2024).
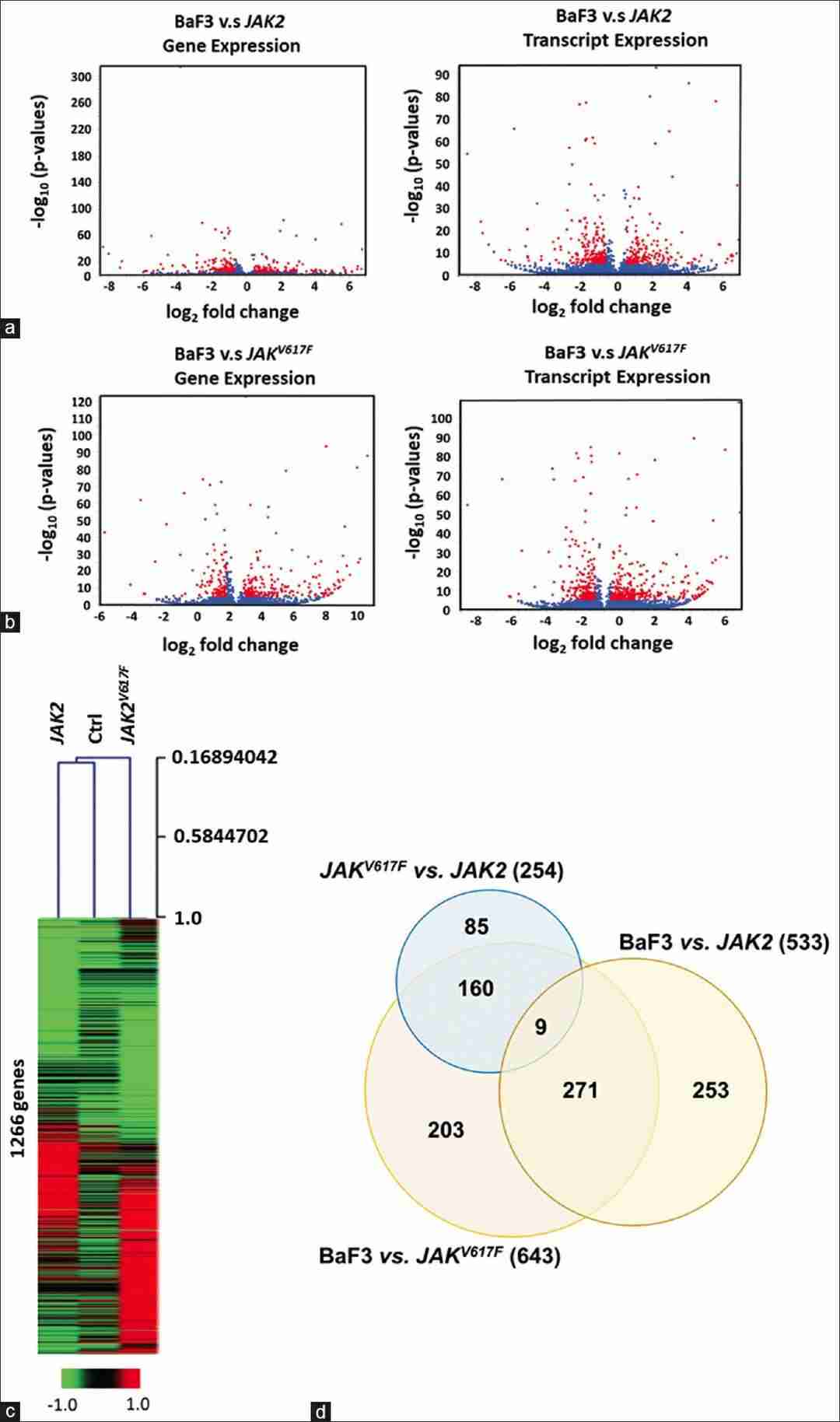 Fig. 2. Identification of the differentially expressed genes/transcripts (Chen Y, Wan Y, et al., 2024).
Fig. 2. Identification of the differentially expressed genes/transcripts (Chen Y, Wan Y, et al., 2024).
Deficiency of Core Fucosylation Activates Cellular Signaling Dependent on FLT3 Expression in a Ba/F3 Cell System
Fms-like tyrosine kinase 3 (FLT3) is a proto-oncogene involved in hematopoiesis, notably mutated in about one-third of acute myeloid leukemia (AML) cases. These mutations, particularly FLT3-ITD and FLT3-TKD, lead to poor prognosis due to abnormal cell signaling. Despite advances, the role of N-glycosylation in FLT3 function remains unclear.
To investigate FLT3's role in inducing core fucosylation, Duan's team created Fut8KO cell lines using murine Ba/F3 cells, typically dependent on IL-3 unless influenced by oncogenes like FLT3-ITD and -TKD. They used the pSpCas9 (BB)-2A-GFP vector for gene editing, establishing stable lines through GFP and PhosL lectin sorting as detailed in "Methods and Materials." Stable Fut8KO cell lines were confirmed via flow cytometry and lectin blotting (Fig. 3A and B), which specifically detects core fucosylated N-glycans. FLT3 is critical for hematopoiesis, influencing proliferation and survival. Testing Fut8KO's effects, we found it increased cell proliferation without IL-3 in FLT3-WT cells (Fig. 4B), unlike control cells. Fut8 restoration returned cells to IL-3 dependency (Fig. 4B). Fut8 ablation partially reduced ITD and TKD cell growth (Fig. 4C and D), suggesting core fucosylation absence strongly activates FLT3-WT.
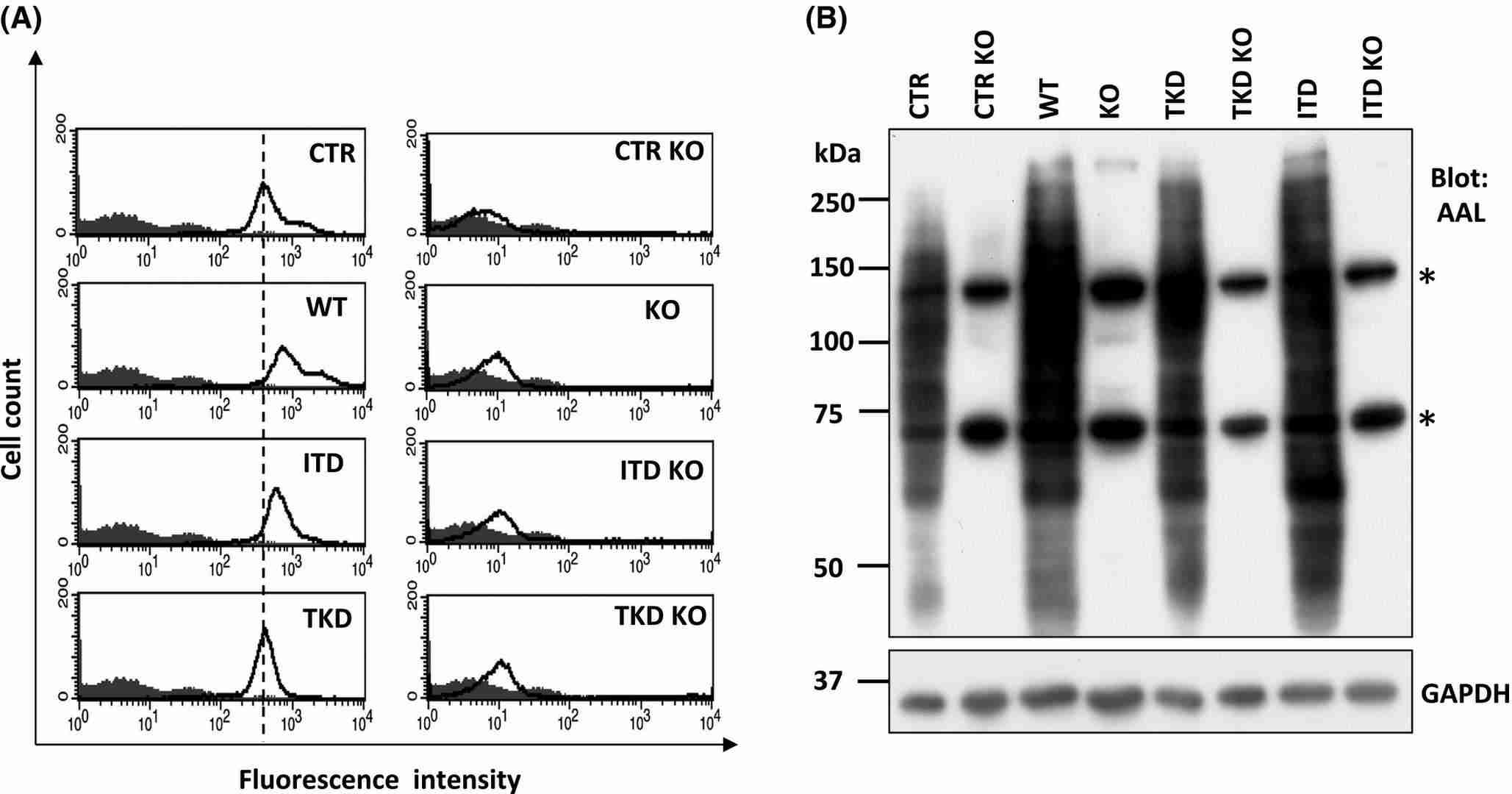 Fig. 3. Established Fut8 deficient Ba/F3 cells (Duan C, Fukuda T, et al., 2020).
Fig. 3. Established Fut8 deficient Ba/F3 cells (Duan C, Fukuda T, et al., 2020).
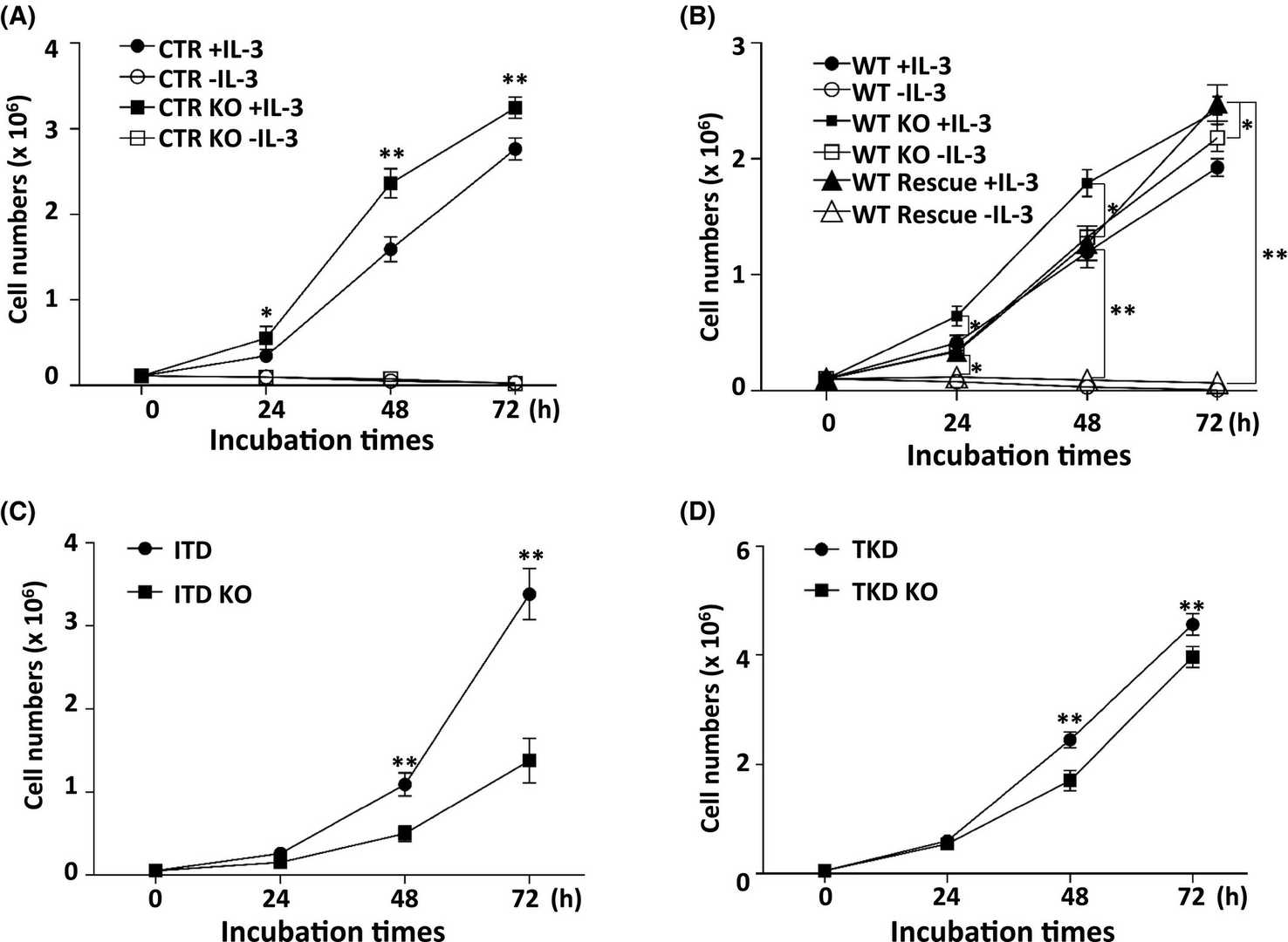 Fig. 4. Influences of core fucosylation on Ba/F3 cell proliferation (Duan C, Fukuda T, et al., 2020).
Fig. 4. Influences of core fucosylation on Ba/F3 cell proliferation (Duan C, Fukuda T, et al., 2020).
Yes, of course. We can provide you with the COA of BA/F3, which includes QC test results of each lot.
Ask a Question
Write your own review
- You May Also Need