Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
Human Neural Progenitor Cells
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Applications:
Drug development
Neurotoxicity
Neurogenesis and CNS function
Neurotransmitter disorders
Electrophysiology
NPCs, human neural progenitor cells, are a type of spindle or oval-shaped multipotent neural stem cell with long cell processes to connect with other neural cells. They are responsible for both the formation and function of the CNS and the PNS. As undifferentiated progenitor cells, NPCs have robust self-renewal processes and are able to convert into other neural cell types, such as neurons, astrocytes and oligodendrocytes. Apart from regenerative replacement, NPCs can also regenerate diseased tissues. They can secrete various bioactive factors that help repair injured but still viable cells, restoring them to normal function.
Such features lend NPCs a convenient template for research on normal and pathological neurobiology, and the design and validation of cell-based neural therapies. To look at disease mechanisms, for instance, NPCs can be used to build models of neurodegenerative disorders (such as multiple sclerosis, Parkinson's disease and Alzheimer's disease) that replicate the course of disease, which can be employed as an avenue for drug discovery and testing. In regenerative medicine and cell therapy, in vitro-cultivated and induced-proliferative NPCs have been routinely implanted or transplanted into patients to treat neurodegeneration and spinal cord injury. Furthermore, a better understanding of how the NPCs are growing, differentiated and migrating biologically could provide insights into neural development processes.
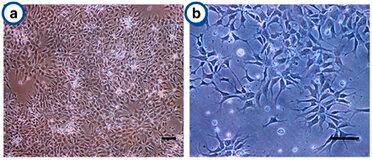 Fig. 1. Cell growth and morphology of the human neural progenitor cells on Antheraea mylitta and mori 3D scaffolds (Bano S., Rao R. R., et al., 2015).
Fig. 1. Cell growth and morphology of the human neural progenitor cells on Antheraea mylitta and mori 3D scaffolds (Bano S., Rao R. R., et al., 2015).
TNF-α Promotes Survival of hNPCs upon OGD Exposure
Neural progenitor cell (NPC) transplantation has been shown to be useful for ischemic brain. However, the low survival rate of transplanted NPCs in the ischemic microenvironment limits its therapeutic effect. Tumor necrosis factor-α (TNF-α) is one of the proinflammatory factors involved in the occurrence of various injuries. Studies have shown that TNF-α affects the proliferation, survival, and differentiation of NPCs.
Kim's team used fetal brain-derived primary human NPCs (hNPCs) to investigate the effects of TNF-α on cell survival under ischemic conditions in vitro. The viability of hNPCs pretreated with various concentrations of TNF-α was evaluated by a CCK-8 assay after OGD exposure (Fig. 1a). hNPC viability was significantly decreased after OGD exposure, and TNF-α pretreatment mitigated the OGD-induced reduction of hNPC viability. Similar trends were noted in LDH assays, which reflected reduced cytotoxicity (Fig. 1b). TUNEL assays demonstrated increased hNPC apoptosis post-OGD, and TNF-α pretreatment reduced apoptosis (Fig. 1c and d). Further experiments focused on 20 ng/ml TNF-α pretreatment due to its significant effect. TNF-α protected hNPCs from OGD-induced viability reduction by mitigating apoptosis and cytotoxicity. Western blotting showed decreased caspase-3 activation in TNF-α pretreated groups, indicating reduced apoptosis (Fig. 1e an f). However, no significant difference was observed in caspase-2 (known as an apoptotic initiator) levels between treated and untreated groups (Fig. 2a and b). In vivo tests showed enhanced engraftment and survival of TNF-α-pretreated hNPCs in HI-injured neonatal mouse brains. Treated groups had more human-specific cell marker-positive cells (STEM121 and STEM101) in the peri-infarct region 3 weeks post-transplantation compared to untreated controls (Fig. 2c-e). These findings confirm TNF-α's protective role in hNPC viability post-OGD by reducing apoptosis and cytotoxicity. Additionally, TNF-α pretreatment improves the survival and engraftment of hNPCs in HI brain injury, suggesting functional recovery potential.
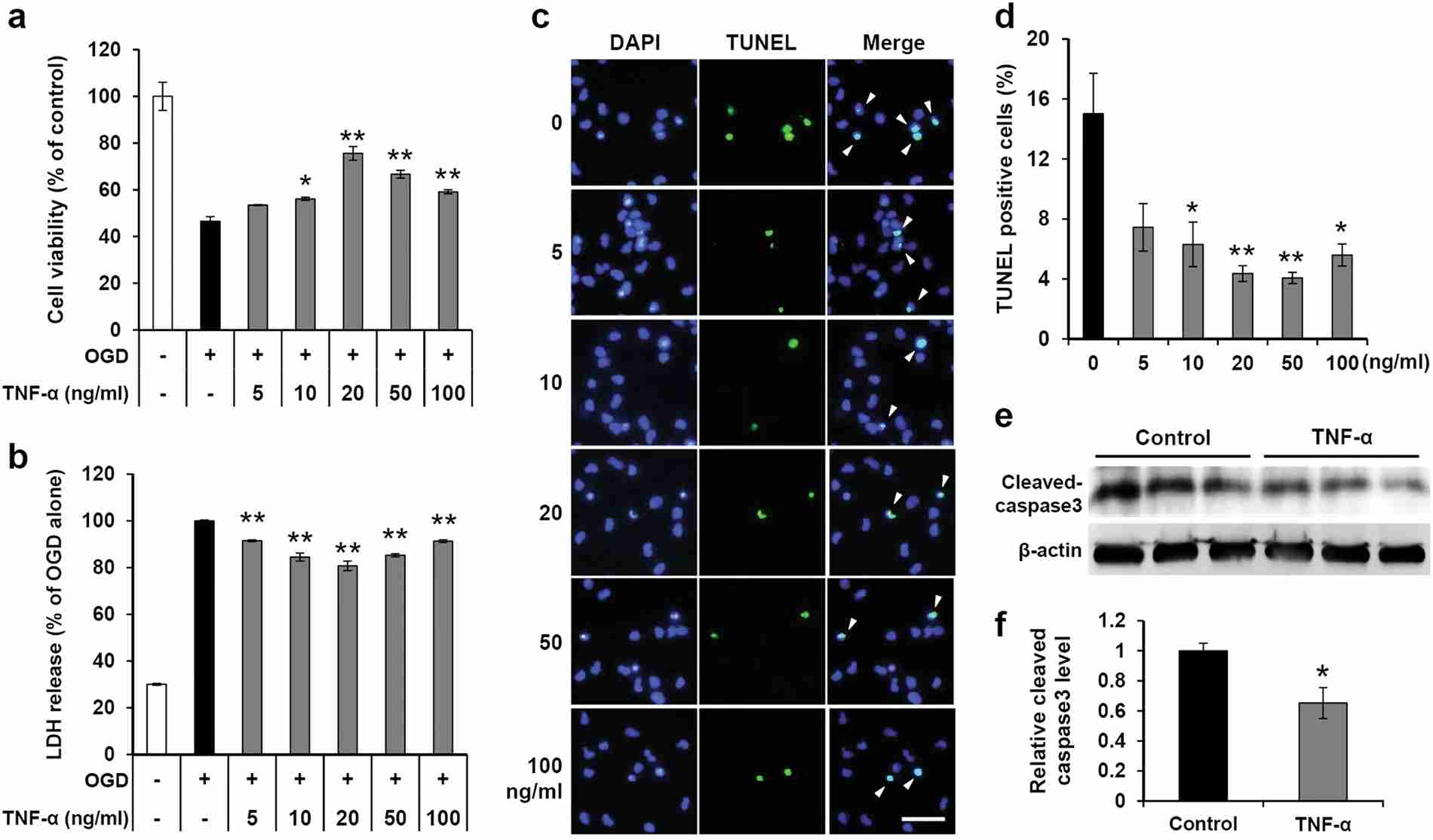 Fig. 1. The protective effect of TNF-α on hNPCs exposed to OGD (Kim, M., Jung, K., et al., 2018).
Fig. 1. The protective effect of TNF-α on hNPCs exposed to OGD (Kim, M., Jung, K., et al., 2018).
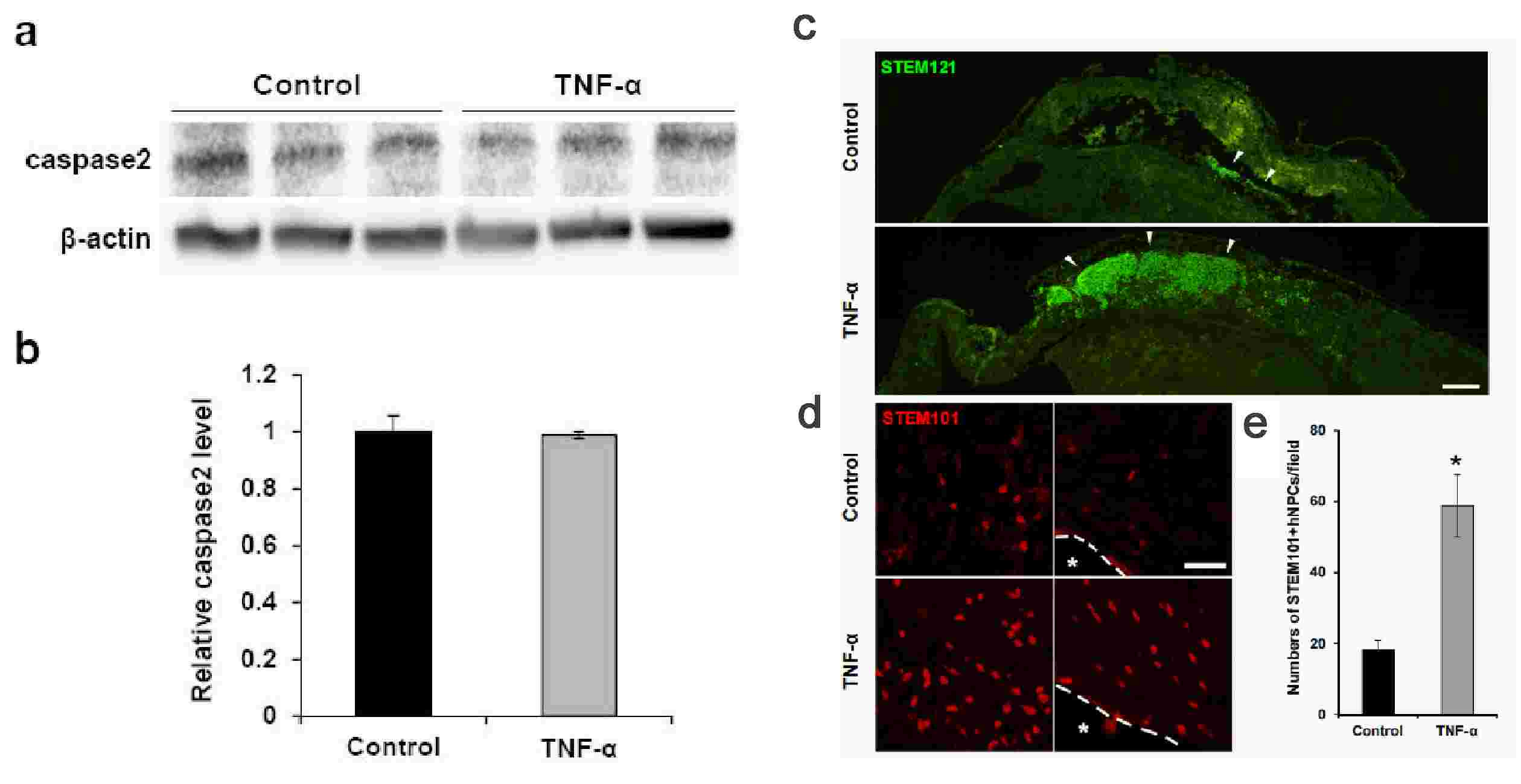 Fig. 2. (a-b) The protein levels of caspase-2 were determined by western blotting. (c-e) Transplantation of TNF-α-pretreated and non-treated hNPCs into HI brain injury in neonatal mouse (Kim, M., Jung, K., et al., 2018).
Fig. 2. (a-b) The protein levels of caspase-2 were determined by western blotting. (c-e) Transplantation of TNF-α-pretreated and non-treated hNPCs into HI brain injury in neonatal mouse (Kim, M., Jung, K., et al., 2018).
PSMD10Gankyrin Levels Increase during Differentiation of hNPCs
PSMD10Gankyrin, a proteasome assembly chaperone, is a widely known oncoprotein which aspects many hall mark properties of cancer. However, except proteasome assembly chaperon function its role in normal cell function remains unknown.
To characterize the effect of PSMD10Gankyrin on overall gene expression, Sahu's team conducted microarray analysis on total RNA isolated from PSMD10Gankyrin-overexpressing stable HEK293 cells. Compared with vector control cells, the analysis identified differentially expressed genes associated with neuronal differentiation, suggesting that PSMD10Gankyrin might regulate cell fate determination. Therefore, they tested the role of PSMD10Gankyrin in neuronal differentiation within human neural progenitor cell (hNPC). After 14 days in media without growth factors (EGF and FGF), hNPCs mainly differentiated into astrocytic cells (~80-90%), with a small portion becoming neurons (~10-15%) (Fig. 3D), and rare oligodendrocyte population observed. In undifferentiated progenitor cells (UD) and after differentiation (DF), PSMD10Gankyrin levels increased significantly in DF cells at both protein (Fig. 3E and F) and mRNA levels (Fig. 3G and H), contrasting with minimal changes in other proteasome subunits (Fig. 3I). Network analyses demonstrated the involvement of nodes like Ngn1 and β-catenin in neurogenesis. In support of this, in PSMD10Gankyrin overexpressing HEK293 cells, an increase in β-catenin protein levels (Fig. 4A), mRNA levels (Fig. 4B), and its transcriptional activity (Fig. 4C) were observed while silencing it reduced them (Fig. 4D and E). Differentiated hNPCs had higher β-catenin protein levels compared to UD cells (Fig. 4F), and increased mRNA levels of Ngn1 and Nrg1 (Fig. 4G and H). Nrg1, crucial for cell growth and differentiation, was upregulated with stable PSMD10Gankyrin but decreased when PSMD10Gankyrin was silenced (Fig. 4D). Monitoring PSMD10Gankyrin during differentiation over 10 days (Fig. 4I-K) revealed its linear increase correlating with β-III tubulin rise, suggesting its role in neuron differentiation.
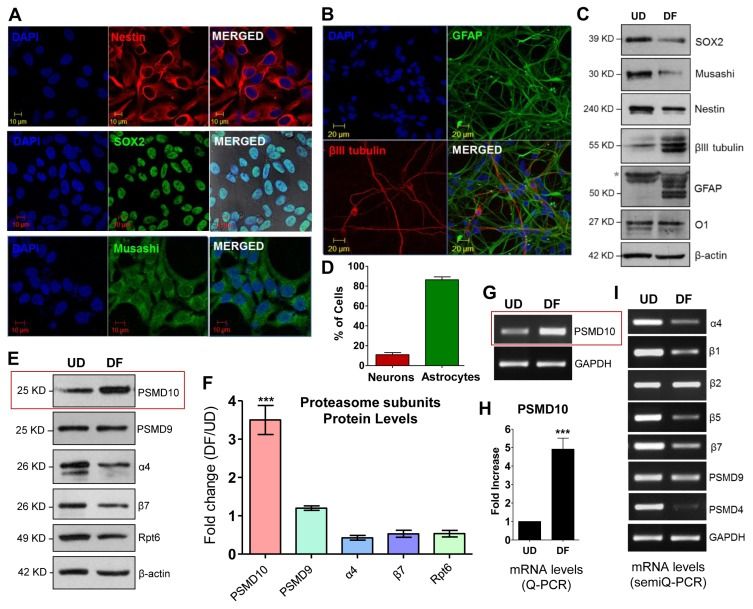 Fig. 3. Differentiated human neural progenitor cells (hNPCs) shows higher expression of PSMD10Gankyrin (Sahu, I., Nanaware, P., et al., 2019).
Fig. 3. Differentiated human neural progenitor cells (hNPCs) shows higher expression of PSMD10Gankyrin (Sahu, I., Nanaware, P., et al., 2019).
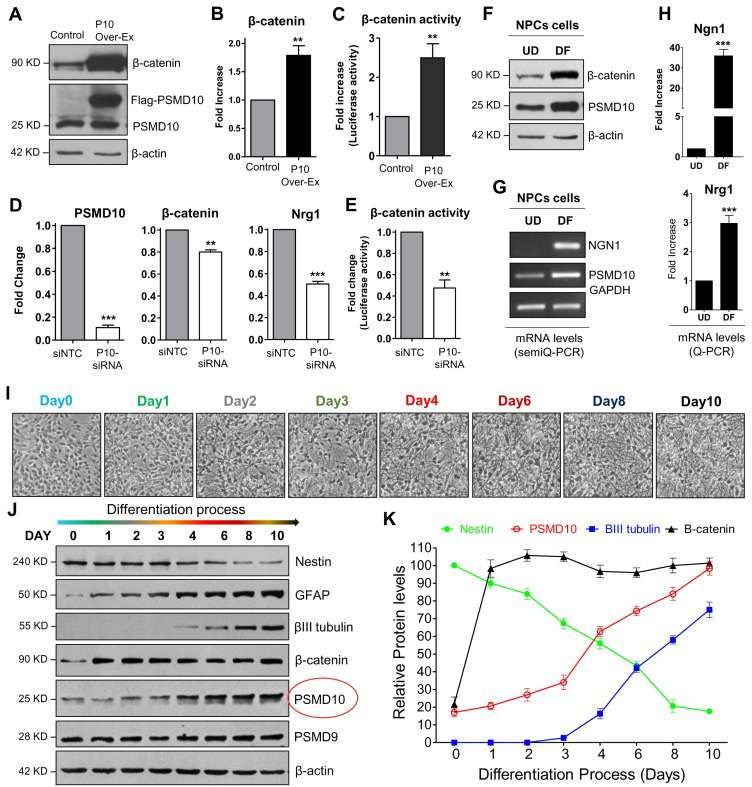 Fig. 4. PSMD10Gankyrin expression increases during the hNPCs differentiation process (Sahu, I., Nanaware, P., et al., 2019).
Fig. 4. PSMD10Gankyrin expression increases during the hNPCs differentiation process (Sahu, I., Nanaware, P., et al., 2019).
Ask a Question
Write your own review
- You May Also Need

