Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY

EMT6/P
Cat.No.: CSC-C9501J
Species: Mouse
Source: Breast
Morphology: Epithelial
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can cryopreserved cells be kept at -20 °C.
The EMT6/P cell line, also known as EMT-6 or Experimental Mammary Tumour-6, is a murine breast carcinoma cell line. The EMT6 cell line was established by transplanting mouse breast cancer into the mammary fat pad of BALB/cCRGL mice, leading to the formation of hyperplastic mammary alveolar nodules. Subsequently, EMT6 cells were isolated from the resulting tumor tissue and propagated in BALB/cKa mice, adapting to tissue culture conditions. The cell morphology of the EMT6/P cell line is epithelial-like.
EMT6/P serves as the parental cell line for a series of drug-resistant cell lines. By inducing resistance in vitro, derived resistant sublines such as EMT6/AR1, EMT6/AR10.0, and EMT6/CPR have been obtained. These provide potent tools for studying the mechanisms of tumor cell resistance and for developing new anti-tumor drugs. Moreover, due to its high tumorigenicity, the EMT6 cell line can form solid tumors in specific BALB/c mouse sub-strains, thus finding extensive application in tumor biology research encompassing tumor growth, metastasis, and therapeutic strategy evaluation. Particularly in breast cancer research, the EMT6/P cell line, serving as an in vitro model, allows the construction of disease models akin to human mammary tumors by engrafting into immunocompromised or immunocompetent mice, thereby providing a crucial experimental foundation for the study and treatment of breast cancer.
Cytotoxicity Study of CeO2@PQQ NPs on Mouse Fibroblasts (L929) and Mouse Adenocarcinoma Cells (EMT6/P)
Ionising radiation, a critical component of cancer radiotherapy, unfortunately induces oxidative stress in cancerous cells as well as normal ones, disrupting vital functions when ROS levels are not properly monitored. The problem has prompted research into novel antioxidants such as cerium oxide nanoparticles (CeO2 NPs), which act as pH-sensitive redox regulators, and pyrroloquinoline quinone (PQQ), which modulates mitochondrial function. Zamyatina's team precipitated CeO2@PQQ NPs, and measured its physical and catalytic characteristics. They then tested the cytotoxicity of cerium oxide-pyrroloquinoline quinone nanoparticles (CeO2@PQQ NPs) against mouse fibroblasts (L929) and mouse adenocarcinoma cells (EMT6/P) to determine non-toxic concentrations for future studies. Based on MTT measurements, they observed that 10 M CeO2@PQQ NPs significantly inhibited NADPH-dependent oxidoreductase activity in L929 cells by 35% after 48 hours; 50 M and 100 M NPs reduced activity by 51% (Fig. 1A). Co-incubation at 0.1 to 2 μM had minimal effects, but at 20 μM, viability sharply decreased by 64%, and by 87% and 81% at 50 and 100 μM, respectively. In EMT6/P cells, metabolic activity remained high up to 20 μM but decreased significantly at higher concentrations (Fig. 1B). A 20 μM concentration reduced metabolic activity for both cell types to the IC50 value, with 50 and 100 μM proving highly toxic even at 48 and 72 hours, decreasing viability by 82% and 96%, respectively.
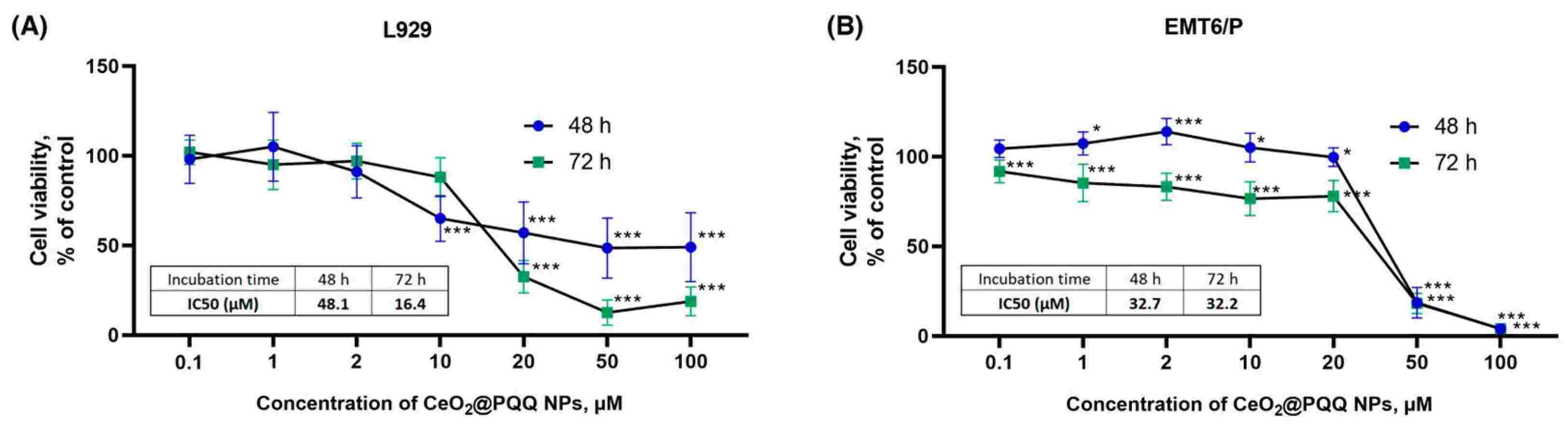 Fig. 1. Cell viability of L929 (A) and EMT6/P (B) cells (Zamyataina EA, Goryacheva OA, et al., 2024).
Fig. 1. Cell viability of L929 (A) and EMT6/P (B) cells (Zamyataina EA, Goryacheva OA, et al., 2024).
Cytotoxicity of Binary Proton Therapy of Mouse Mammary Carcinoma Cells (EMT6/P line) Using Targeted Gold Nanoparticles
Proton therapy is an advanced technique in radiation oncology due to its precision in targeting tumors located in radiation-sensitive tissues. However, delivering nanoparticles effectively to the tumor site remains challenging. A promising target for targeting is the FRα isoform of the folate receptor. Therefore, Filimonova's team aims to explore the use of gold nanoparticles, specifically Au-PEG-FA nanoparticles, to enhance the efficacy of proton therapy by targeting the folate receptor. To assess the enhancement of proton therapy with nanoparticles, cytotoxicity was tested on mouse mammary carcinoma cells (EMT6/P line). Cells were treated with gold nanoparticles and incubated for 16 hours before irradiation at the Prometheus proton therapy complex in Russia, with a proton energy of 160.5 MeV and beam homogeneity of at least 98% at 95% isodose. Eight days post-irradiation, a significant reduction in clonogenic activity was observed with binary therapy (Fig. 2). Au-PEG nanoparticles (10–50 μg/mL) did not reduce cell colony formation, indicating high biocompatibility. Cells showed a dose-dependent reduction in colonies by 25% and 50% at 2 and 4 Gy, respectively. Preincubation with 50 μg/mL Au-PEG and 2 Gy co-irradiation resulted in a fourfold colony decrease versus non-irradiated controls (Fig. 2). Preincubation with 25 or 50 μg/mL nanoparticles and 4 Gy irradiation led to complete cancer colony elimination, demonstrating Au-PEG's biocompatibility and radiosensitizing effect at 2–4 Gy in vitro.
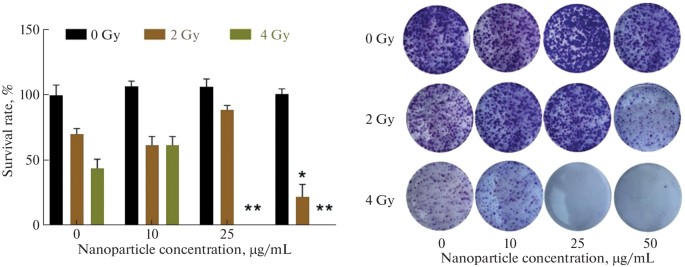 Fig. 2. Clonogenic analysis of EMT6/P adenocarcinoma cells after proton beam irradiation in the presence of Au-PEG nanoparticles (Filimonova MV, Kolmanovich DD, et al., 2024).
Fig. 2. Clonogenic analysis of EMT6/P adenocarcinoma cells after proton beam irradiation in the presence of Au-PEG nanoparticles (Filimonova MV, Kolmanovich DD, et al., 2024).
Ask a Question
Write your own review