Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY

EMT6/AR1
Cat.No.: CSC-C9497J
Species: Mouse
Source: Breast
Morphology: Epithelial
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can cryopreserved cells be kept at -20 °C.
The EMT6/AR1 cell line, a crucial variant derived from the murine mammary carcinoma cell line EMT6/P, was obtained as a resistant strain under laboratory conditions through selective pressure by gradually increasing concentrations of Adriamycin (Doxorubicin). This process simulates the development of drug resistance by tumor cells during clinical chemotherapy, providing a valuable model for research.
Compared to the parental cell line EMT6/P, EMT6/AR1 demonstrates up to 40-fold resistance to Adriamycin and exhibits a typical multidrug resistance (MDR) phenotype. This significant MDR trait is primarily attributed to the overexpression of P-glycoprotein. P-glycoprotein, a transmembrane protein, extrudes various chemotherapeutic substances out of cells to decrease drug levels within the cell, and cause drug resistance. EMT6/AR1 cells are also highly resistant to some drugs like doxorubicin and docetaxel. These cells express many MDR proteins such as P-glycoprotein, and this has made them a valuable resource to study the structure and function of those proteins, as well as drug transport and resistance. It helps to identify targeted inhibitors of these proteins that would enhance the effectiveness of cancer chemotherapy. EMT6/AR1 cells possess an epithelial structure and adherent growth properties and have the potential to develop solid tumors in vivo and in vitro. As a result, EMT6/AR1 cells are also used to screen for new antitumor drugs that overcome Adriamycin and multidrug resistance, and provide an experimental platform for newer cancer therapies.
Determining ABC Transporter Expression and Cell Motility in Drug-Resistant TNBC
Triple-negative breast cancer (TNBC), which lacks estrogen, progesterone, and HER2 receptors, is highly aggressive and recurrent. Chemotherapy is the primary treatment but drug resistance, in this case against doxorubicin (Dox), prevents it from working. To combat this, Hsiao et al. seeks to enhance treatment effectiveness in the face of drug-resistant TNBC through a new nanocarrier system based on biodegradable polymers that delivers targeted drug delivery. In drug resistance mechanisms, ABC transporter activation causes increased drug efflux, leading to cancer drug resistance. They studied ABC transporter expression in three TNBC cell lines (EMT6, EMT6/AR1, and EMT6/AR10) with varying drug resistance. ABCB1 levels rose 1.5-fold in EMT6/AR1 cells and 1.8-fold in EMT6/AR10 cells, while ABCG2 levels rose 1.3-fold and 1.4-fold, respectively. ABCC1 levels were unchanged across the cell lines (Fig. 2A). P-selectin was upregulated around breast cancer cells, with consistent expression found in the TNBC lines (Fig. 2A), suggesting no link to drug resistance. Additionally, P-selectin expression in non-transformed mammary gland cells (NMuMG) was assessed, showing a 2.4-fold increase in EMT6/AR1 cells compared to NMuMG cells (Fig. 1). Migration and invasion studies revealed EMT6/AR1 and EMT6/AR10 cells had heightened abilities compared to EMT6, especially in EMT6/AR1 cells (1.84-fold in migration and 2.20-fold in invasion) (Fig. 2B). These results confirmed that drug resistance was correlated with cell motility.
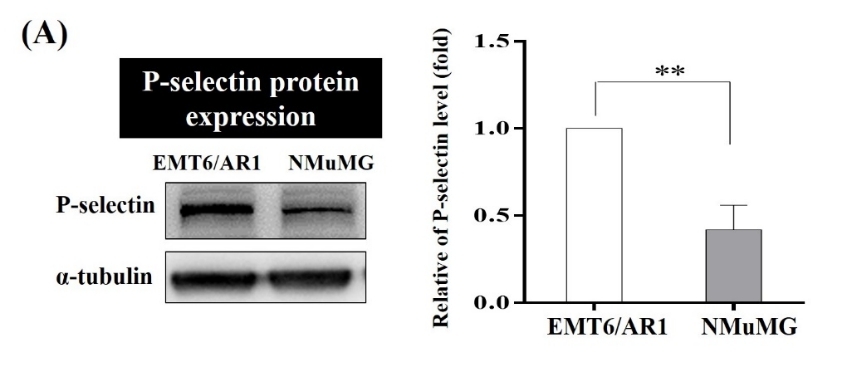 Fig. 1. P-selectin level of EMT6-AR1 and NMuMG cells (Hsiao C, Huang H, et al., 2022).
Fig. 1. P-selectin level of EMT6-AR1 and NMuMG cells (Hsiao C, Huang H, et al., 2022).
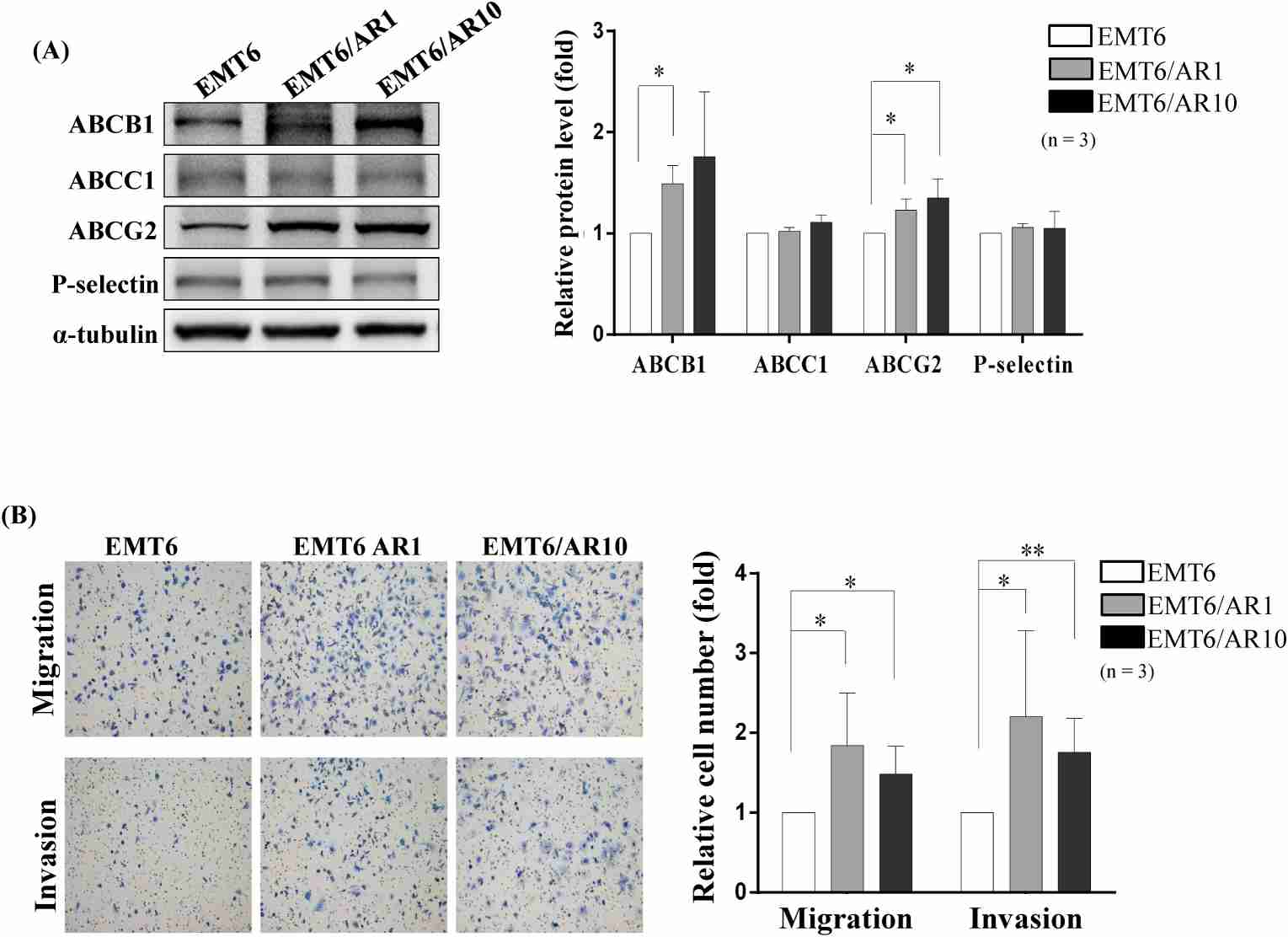 Fig. 2. Expression of the ABC transporter family and P-selectin proteins in mouse mammary carcinoma cell lines and their motility pattern (Hsiao C, Huang H, et al., 2022).
Fig. 2. Expression of the ABC transporter family and P-selectin proteins in mouse mammary carcinoma cell lines and their motility pattern (Hsiao C, Huang H, et al., 2022).
VDNPs Enhances Uptake, and Retention of pDox in MDR EMT6/AR-1
Multi-drug resistance (MDR) in cancer is mainly due to overexpressed drug efflux pumps and mitochondrial activity, making cancer mitochondria a prime target. Previous studies identified VES-H8R8, a mitochondrial-targeting compound, effective in depolarizing mitochondria and inhibiting drug efflux in MDR cancer cells. Czupiel's team synthesized and validated a pH-sensitive doxorubicin prodrug (pDox) for release under acidic conditions. VES-H8R8 and pDox were co-encapsulated in nanoparticles (NPs) and tested against parental breast cancer cell line, EMT6/P, and the MDR breast cancer cell line, EMT6/AR-1. The anti-cancer activity of pDox and VES-H8R8 were assessed by the Presto Blue metabolic assay, and results showed VDNPs synergistically increase anti-cancer activity in a MDR breast cancer model. To explore VDNPs' synergistic mechanism, they assessed the uptake of free Dox, DNPs, and VDNPs in MDR EMT6/AR-1 cells over 2, 5, and 24 hours using confocal microscopy (Fig. 3A). Free Dox showed minimal fluorescence, highlighting active drug efflux. In contrast, DNP and VDNP treatments revealed endo-lysosomal localization and cytosolic and nuclear fluorescence at 24 hours, indicating effective delivery and Dox release. Both DNPs and VDNPs showed increasing Dox accumulation over time, with VDNPs exhibiting superior uptake. This was evidenced by diffuse cytoplasmic fluorescence, suggesting endosomal escape. Flow cytometry further quantified this, with VDNPs significantly outperforming DNPs and free Dox in delivering Dox into MDR cells (Fig. 3B). After 24 hours, VDNPs achieved a 2.4-fold higher mean fluorescence intensity (M.F.I) compared to DNPs, confirming enhanced retention and uptake, while free Dox showed minimal fluorescence due to active efflux.
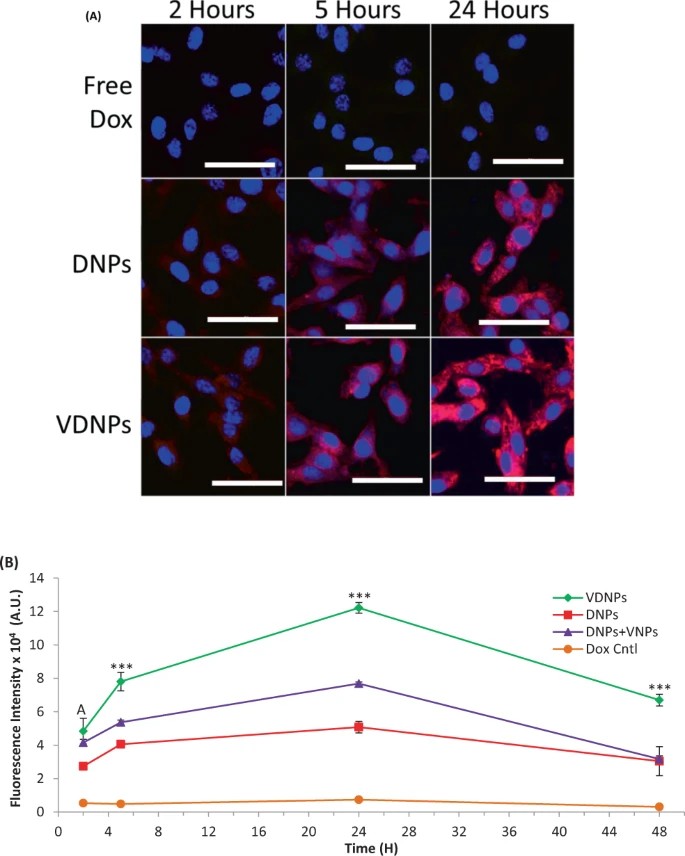 Fig. 3. Multi-drug resistant (MDR) breast cancer cells treated with doubly loaded nanoparticles (NPs) show a significant increase in doxorubicin (Dox) accumulation and retention compared to controls (Czupiel P, Delpace V, et al., 2020).
Fig. 3. Multi-drug resistant (MDR) breast cancer cells treated with doubly loaded nanoparticles (NPs) show a significant increase in doxorubicin (Dox) accumulation and retention compared to controls (Czupiel P, Delpace V, et al., 2020).
Ask a Question
Write your own review