Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
Dental Pulp Stem Cells Adult Teeth
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Dental Pulp Stem Cells (DPSCs) are fibroblast cells isolated from the pulp tissue of human teeth and are a form of mesenchymal stem cell. These cells mostly come from natural shedding teeth or removed wisdom teeth. Since stem cell acquisition from tooth extraction is less invasive than extracting cells from other tissues, adult DPSCs are a convenient stem cell source. DPSCs express surface mesenchymal stem cell antigens, including CD90+, CD146+, CD105+ and CD45; pluripotency markers, including Oct4 and Nanog, although not in the concentrations seen with SHED cells.
The remarkable property of adult dental pulp stem cells is that they are pluripotent. They can differentiate into odontoblasts (dentine cells), osteoblasts (bone-building cells), adipocytes and neuronal cells. This pluripotency makes them highly attractive candidates for tissue repair and regeneration. Adult dental pulp stem cells can self-renew, that is, divide and generate offspring with the same properties and possibilities as the parental cells. This capability ensures a constant flow of stem cells throughout the pulp, and allows them to be multiplied in vitro for studies and treatment.
Clinically, they have been studied to treat a large array of connective tissue, skeletal and neurological disorders. DPSCs have shown immunomodulatory effects, making them a potential anti-autoimmune treatment. And, because of their neural crest origin, DPSCs are particularly useful in neurodegenerative conditions, and have been found to induce neovascularization after ischemic brain injury, dopamine stimulation during Parkinson's disease, and inhibition of astrocyte activation following spinal cord injury.
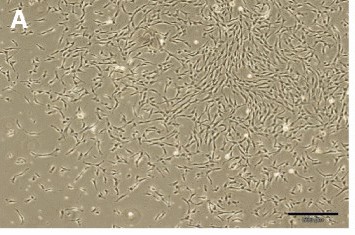 Fig. 1. Dental pulp stem cells (DPSCs). In primary cultures, the cells formed large colonies that consisted of fibroblastic cells (Bakhtiar H, Mirzaei H., et al., 2017)
Fig. 1. Dental pulp stem cells (DPSCs). In primary cultures, the cells formed large colonies that consisted of fibroblastic cells (Bakhtiar H, Mirzaei H., et al., 2017)
Electrophysiological Function of Induced Neural-like Cells Via Neurosphere-Mediated Approach
Due to the limited sources of human neural stem/progenitor cells for neural tissue regeneration, Li's team turned their attention to mesenchymal stromal/stem cells (MSCs), including oral stem cells (OSCs) derived from cranial neural crest cells. Induced oral and non-oral stem cells have been studied for their neurogenic potential. Induced dental pulp stem cells (DPSCs), gingiva-derived mesenchymal stem cells (GMSCs), generally demonstrated greater neurogenic potential in terms of morphology and neurogenic gene expression levels compared to other cells. Consequently, electrophysiological testing was performed on induced DPSCs and GMSCs.
They selected cells that had a neuron-like morphology as test subject and Whole-cell patch-clamp to measure voltage-gated Na+ currents and K+ currents. The results showed that, both voltage-dependent Na+ and K+ currents in DPSC-neuronal cells and GMSC-neuronal cells, but not in non-induced cells (Fig. 1a, b; Fig. 2a, b). These inward Na+ currents could be completely blocked upon perfusion of 1 μM TTX and rapidly recovered with wash of TTX. Similarly, the K+ currents could be completely and reversely blocked by 35 mM TEA (Fig. 1c–f and 2c–f). Therefore, the electrophysiological response seen in DPSCs and GMSCs is mainly due the opening and closing of voltage-gated Na+ and K+ channels since the currents were blocked by TTX and TEA. Of the patched cells, 8.3% of DPSC-derived neurons and 21.4% of GMSC-derived neurons showed action potentials (AP), with GMSC-derived neurons also having a higher percentage of detectable Na+ and K+ currents (DPSCs: Na+ 27.1%, K+ 37.5%; GMSCs: Na+ 67.3%, K+ 65.4%). When considering donors, AP-positive percentages were slightly higher (DPSCs: 20%; GMSCs: 36.4%). No spontaneous APs were observed. The AP peak was smaller and repolarization was incomplete, with Na+ currents between 150 and 400 pA, requiring hyperpolarization for multiple APs per depolarization.
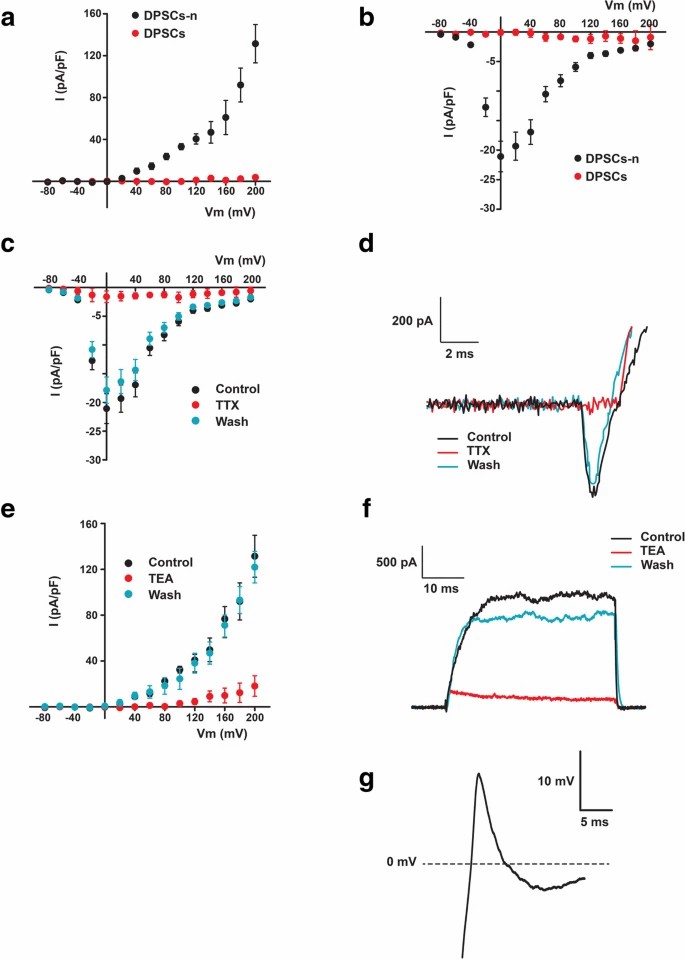 Fig. 1. Neuronal cells derived from DPSCs (DPSCs-n), but not the unstimulated DPSCs, displayed voltage-dependent K+ and Na+ currents that evoked action potentials. (Li D, Zou XY, et al., 2019).
Fig. 1. Neuronal cells derived from DPSCs (DPSCs-n), but not the unstimulated DPSCs, displayed voltage-dependent K+ and Na+ currents that evoked action potentials. (Li D, Zou XY, et al., 2019).
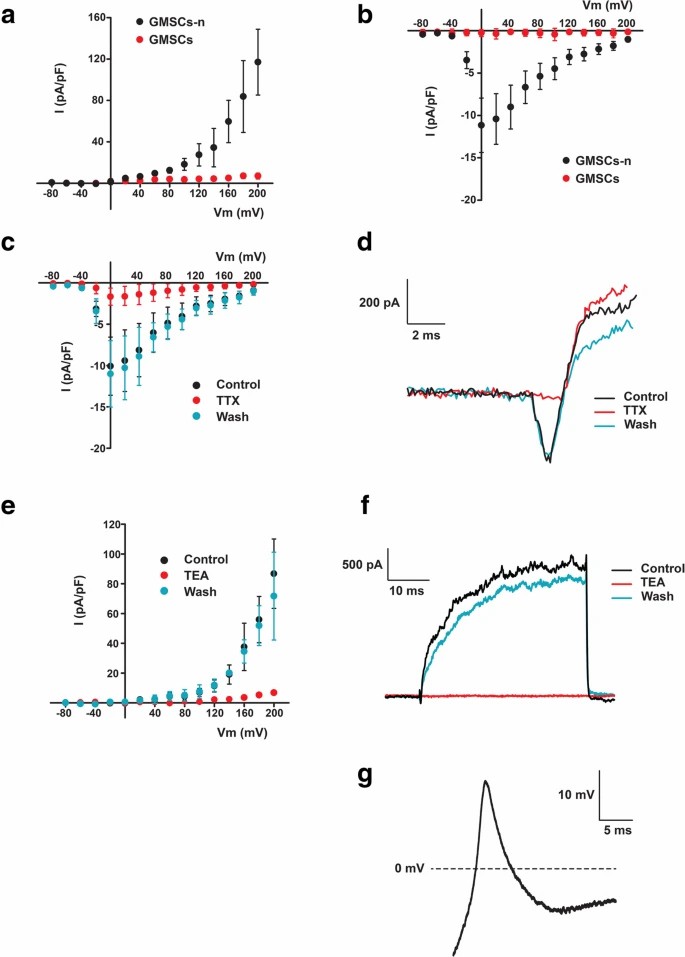 Fig. 2. Neuronal cells derived from GMSCs (GMSCs-n), but not unstimulated GMSCs, displayed voltage-dependent K+ and Na+ currents that evoked action potentials (Li D, Zou XY, et al., 2019).
Fig. 2. Neuronal cells derived from GMSCs (GMSCs-n), but not unstimulated GMSCs, displayed voltage-dependent K+ and Na+ currents that evoked action potentials (Li D, Zou XY, et al., 2019).
hDPSCs Acquired Neuronal-Like Morphological Features after Neuronal Induction
Human dental pulp stem cells (hDPSCs) are gaining attention for nerve regeneration, yet their neurogenic potential is debated. Stem cells' capability to differentiate into neurons makes them potential candidates for nerve repair, especially in areas like dentistry. Current neuron differentiation methods from hDPSCs often involve complex, time-consuming protocols. However, a simpler method using all-trans retinoic acid (ATRA) and brain-derived neurotrophic factor (BDNF) has been proven effective for SH-SY5Y cells, a model for neurological studies. Al-Maswary et al. explored their effectiveness in hDPSC induction.
The hDPSCs and SH-SY5Y cells demonstrated comparable results that both showed significant transformation into neuronal-like cells using the sequential supplementation method with ATRA followed by BDNF (Fig. 3A–L). SH-SY5Y cells developed fine neurite extensions after ATRA treatment (Fig. 3B), which further branched into a multipolar neuronal-like morphology after BDNF supplementation (Fig. 3D). Without BDNF, control cells lost their neurite phenotype (Fig. 3C). Similarly, hDPSCs displayed bipolar elongation post-ATRA treatment (Fig. 3F) and acquired defined bipolar and multipolar neuronal-like morphologies after BDNF (Fig. 3H–L). Typical morphologies are shown in Figure 3K (bipolar) and Figure 3L (multipolar). Control hDPSCs without BDNF showed no neuronal transformations (Fig. 3G). These results affirm the effectiveness of ATRA followed by BDNF for inducing neuronal characteristics.
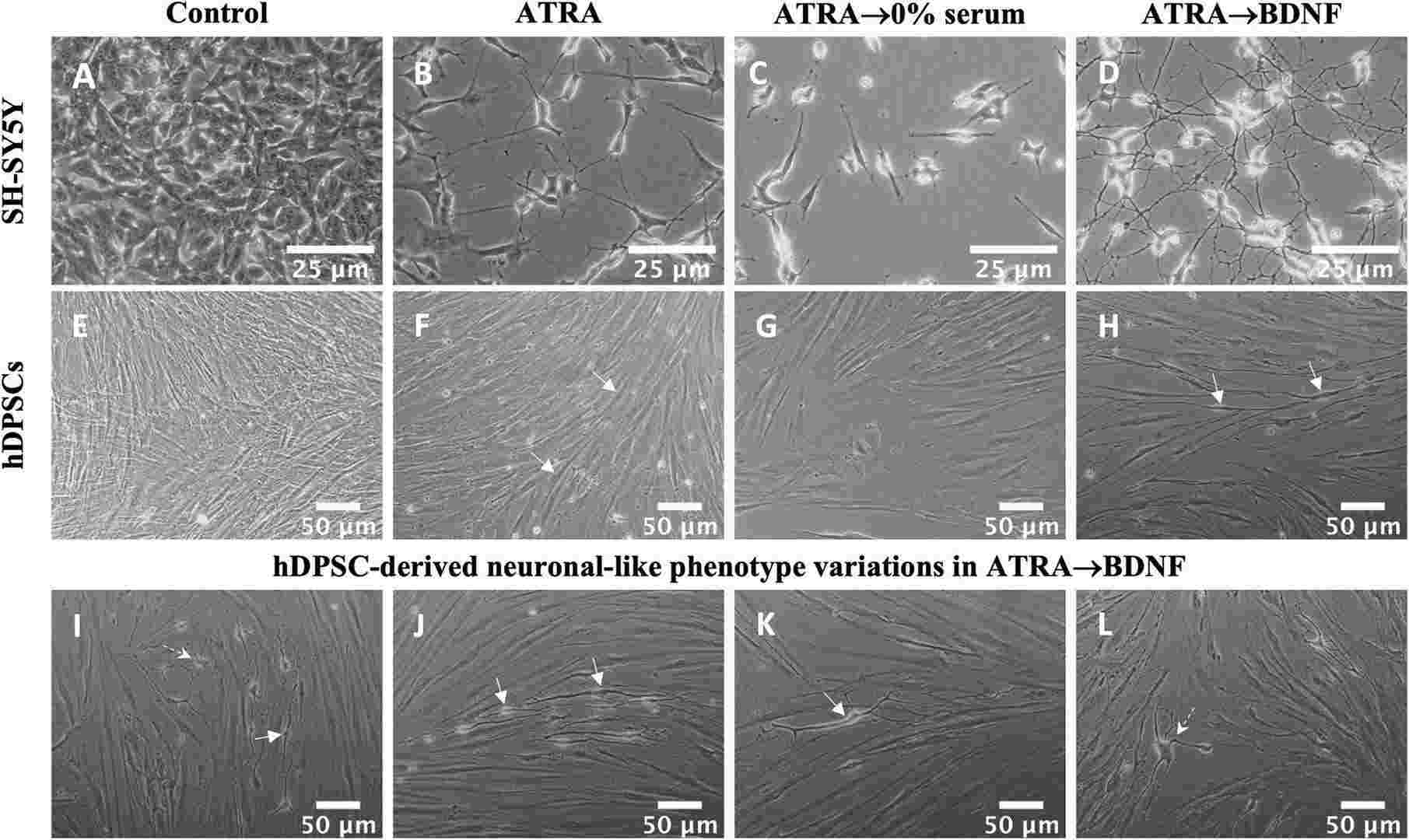 Fig. 3. Neural differentiation of SH-SY5Y and hDPSC experimental groups (Al-Maswary AA, O'Reilly M, et al., 2022).
Fig. 3. Neural differentiation of SH-SY5Y and hDPSC experimental groups (Al-Maswary AA, O'Reilly M, et al., 2022).
Ask a Question
Write your own review

