Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY

DEL
Cat.No.: CSC-C0397
Species: Human
Source: malignant histiocytosis
Morphology: grows often in clusters in suspension, most cells are polygonal (some cells are round)
Culture Properties: suspension
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Immunology: CD3 -, CD7 -, CD10 -, CD14 -, CD15 +, CD19 -, CD25 +, CD30 +, CD34 -
Viruses: ELISA: reverse transcriptase negative; PCR: EBV -, HBV -, HCV -, HHV-8 -, HIV -,
DEL cell line is derived from the pleural effusion of a 12-year-old boy with malignant histiocytosis in 1987 (possibly anaplastic large cell lymphoma, ALCL). This cell line tends to grow in suspension in culture. This DEL cell line contains the NPM1-ALK (NPM-ALK) fusion gene that was created by a chromosomal translocation, which is a common genetic disorder of ALCL in children and adults. The NPM1 (nucleophosmin) and ALK (anaplastic lymphoma kinase) proteins fusion into an oncogenic fusion protein. NPM-ALK drives ALCL pathogenesis by triggering various signaling pathways including JAK/STAT and PI3K/AKT/mTOR which are important for cell growth, survival and other oncogenes. For example, NPM-ALK positive tumor cells require ongoing activation of the JAK3/STAT3 pathway to continue to exist.
With the NPM-ALK fusion gene, DEL cell line is an invaluable resource for understanding ALCL pathology, disease progression and therapeutic targets. Scientists use this cell line for high-throughput drug testing and functional genetic analysis to discover inhibitors that block the NPM-ALK fusion protein that might be therapeutically useful for ALCL. In addition, genetic studies could explain the oncogenic activity of NPM1-ALK. Moreover, the DEL cell line can be used to study ALCL cellular biology, drug tolerance, and relationships with other cell lines.
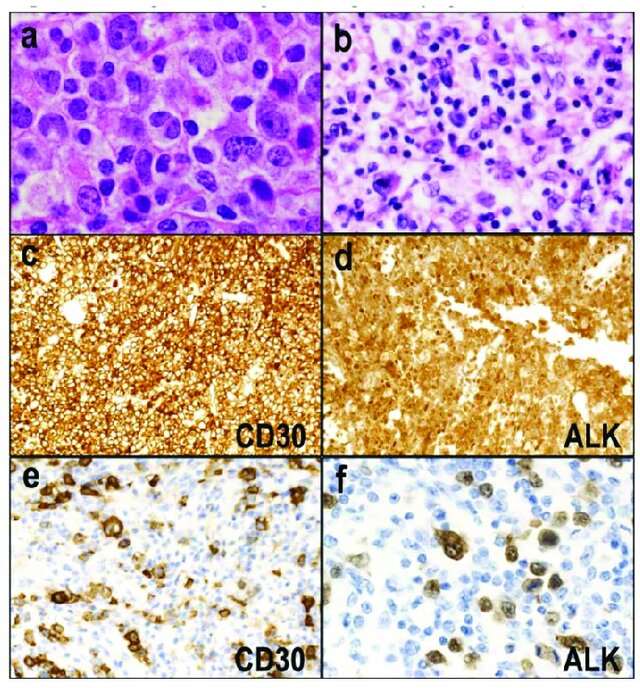 Fig. 1. ALK-positive anaplastic large cell lymphoma (ALCL) (Satou, A., Takahara, T., et al., 2022).
Fig. 1. ALK-positive anaplastic large cell lymphoma (ALCL) (Satou, A., Takahara, T., et al., 2022).
SQLE Deficiency and Cholesterol Auxotrophy in ALK+ ALCLs
This study explores the cholesterol dependency of cancer cells, particularly in ALK+ anaplastic large cell lymphoma (ALCL) cell lines (ALK+ ALCLs). Garcia-Bermudez et al. discovered that these cells rely heavily on cholesterol uptake due to a loss of squalene monooxygenase (SQLE), identifying the low-density lipoprotein receptor (LDLR) as crucial for their growth, presenting a potential therapeutic target. Using gene expression data from the cancer cell line encyclopedia (CCLE), Garcia-Bermudez et al. identified nine cell lines lacking SQLE mRNA and protein. Remarkably, six of these belong to the ALK+ ALCLs: DEL, SU-DHL-1, KIJK, SUP-M2, SR-786, and Karpas 299 (Fig. 1d and e). Similar to SNU-1, these SQLE-deficient ALCL cell lines are sensitive to cholesterol depletion and accumulate high levels of squalene (Fig. 1g and f). SRS microscopy revealed that ALK+ALCLs accumulate squalene in lipid droplets (Fig. 1h). The loss of SQLE expression is linked to promoter hypermethylation, with DNA-hypomethylating agents upregulating SQLE mRNA. Primary tumor samples and patient-derived xenografts of ALK+ ALCLs also showed reduced SQLE expression. Despite the inverse correlation between NPM-ALK translocation and SQLE expression, ALK signaling modulation did not affect SQLE expression, suggesting a different silencing mechanism (Fig. 2). These findings indicate that ALK+ ALCLs lack SQLE expression, making them cholesterol auxotrophs.
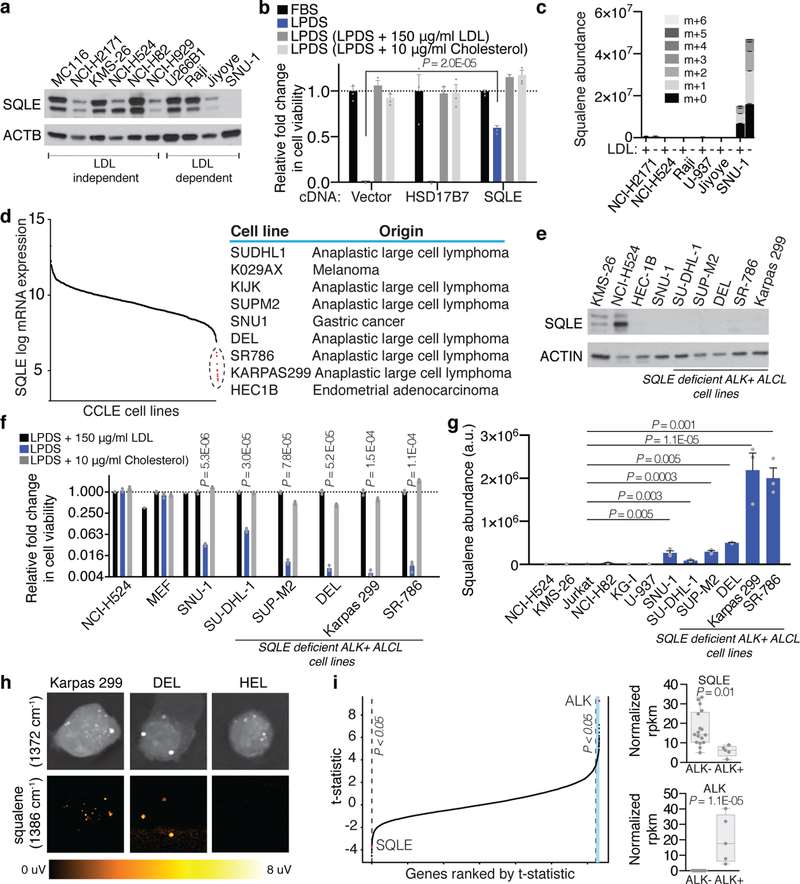 Fig. 1. ALK+ ALCLs are auxotrophic for cholesterol due to lack of SQLE expression (Garcia-Bermudez, J., Baudrier, L., et al., 2019).
Fig. 1. ALK+ ALCLs are auxotrophic for cholesterol due to lack of SQLE expression (Garcia-Bermudez, J., Baudrier, L., et al., 2019).
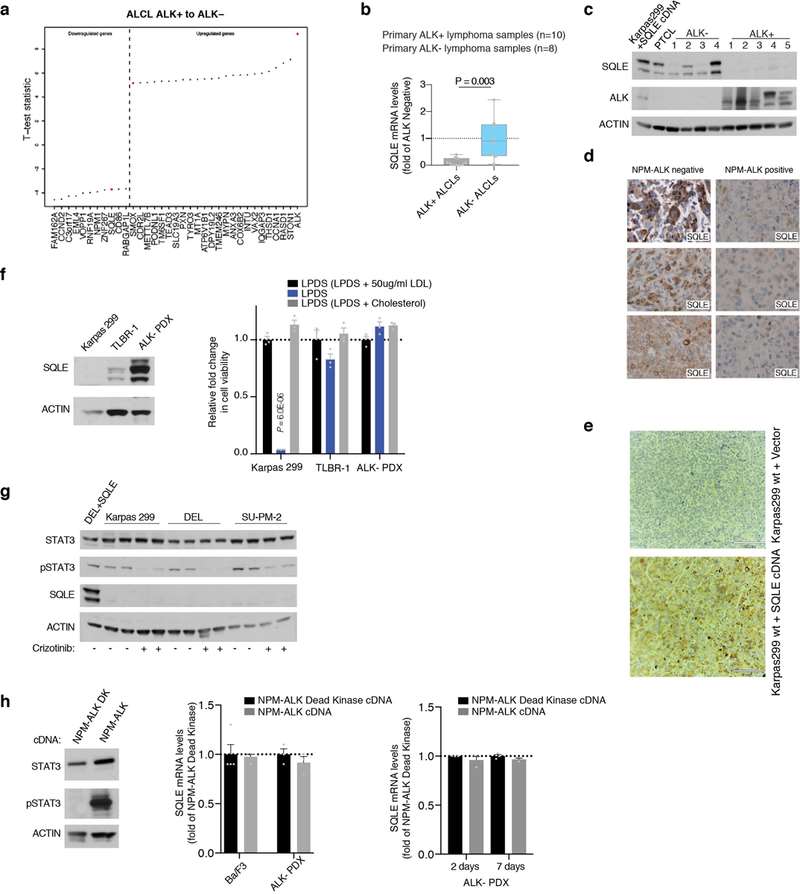 Fig. 2. Lack of SQLE expression in primary ALK+ ALCLs (Garcia-Bermudez, J., Baudrier, L., et al., 2019).
Fig. 2. Lack of SQLE expression in primary ALK+ ALCLs (Garcia-Bermudez, J., Baudrier, L., et al., 2019).
ALK Inhibitor Crizotinib Reduces ACLY Y682 Phosphorylation in ALK+ Lymphoma and Neuroblastoma Cells
Rapid cell proliferation requires metabolic adaptation for biomass synthesis, with ATP citrate lyase (ACLY) playing a crucial role in linking carbohydrate and lipid metabolism. Basappa J et al. investigated the role of tyrosine phosphorylation at Y682 in enhancing ACLY activity and its oncogenic implications in multiple cancers. Phosphoproteomics analysis of lymphoma cell lines with NPM-ALK fusion tyrosine kinase identified 881 phosphorylated tyrosine peptides. Key findings indicate that ACLY Y682 phosphorylation significantly correlates with ALK Y1604 activation. ALK inhibition via CEP-26939 reduced ACLY Y682 phosphorylation, confirmed by Crizotinib (an ALK inhibitor) treatment (Fig. 3f). They treated two ALK+ ALCL cell lines (SUDHL-1, DEL) and one neuroblastoma cell line (NB1) with Crizotinib, observing reduced Y682 phosphorylation through anti-pACLY Y682 antibody immunoblotting (Fig. 3a). Immunohistochemistry on biopsies from ALK+ and ALK- ALCL patients showed a strong correlation between ACLY Y682 phosphorylation and ALK expression (Fig. 3b). Creating an ALK+ ALCL cell line (DEL) with either an empty vector, HA-tagged ACLY-WT, or ACLY-Y682F, immunoblotting revealed increased Y682 phosphorylation in ACLY-WT but not in ACLY-Y682F cells (Fig. 3c). Additionally, transducing primary human T cells with either active NPM-ALK or kinase-deficient NPM-ALK-K210R showed ACLY Y682 phosphorylation only in cells with active NPM-ALK (Fig. 3e).
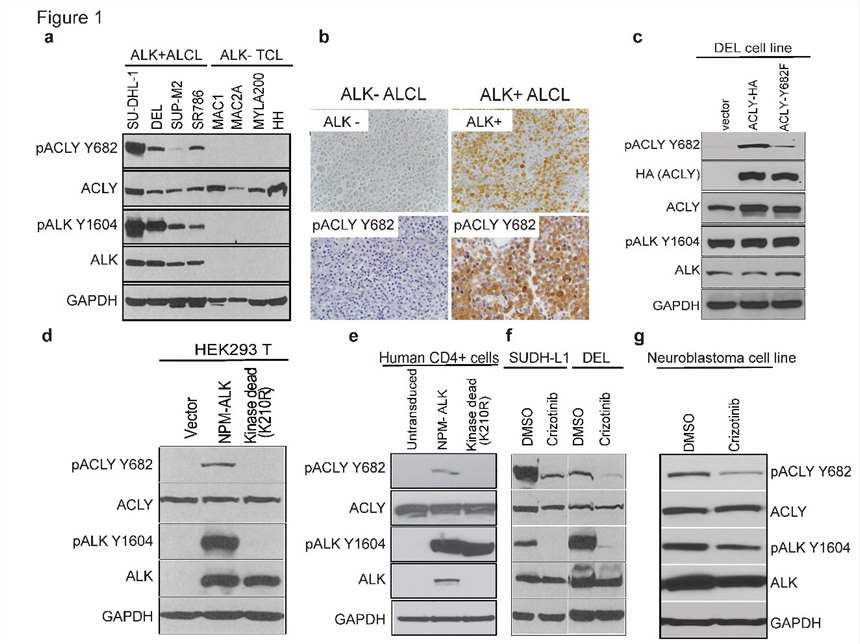 Fig. 3. NPM-ALK interacts with ACLY and phosphorylates on Y682 residue in ALK+ALCLs (Basappa J., ElAzzouny, M. A., et al., 2020).
Fig. 3. NPM-ALK interacts with ACLY and phosphorylates on Y682 residue in ALK+ALCLs (Basappa J., ElAzzouny, M. A., et al., 2020).
Interphase Fluorescence In Situ Hybridization (FISH) Multiple myeloma FISH panel aids in stratifying individuals with newly diagnosed multiple myeloma into risk groups for prognosis and selection of therapy. It is also useful in following up on remission or relapse status.
Ask a Question
Average Rating: 4.0 | 1 Scientist has reviewed this product
Well-characterized
These cells were well characterized and matched the description given in the instructions.
23 Feb 2023
Ease of use
After sales services
Value for money
Write your own review
- You May Also Need