Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
A2780/cis
Cat.No.: CSC-C9492J
Species: Human
Source: Reproductive: Ovary
Morphology: Epithelial
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can cryopreserved cells be kept at -20 °C.
The A2780/cis cell line is a specially screened and cultured human ovarian cancer cell line. It was developed by exposing parental cisplatin-sensitive A2780 cells to progressively increasing concentrations of cisplatin for prolonged time and thus acquired resistance to cisplatin. Such resistance comes from abnormal encoding of drug transporter proteins, improved repair of DNA damage, and so on. This resistance requires that cisplatin be introduced into the culture medium every 2-3 generations to sustain it. Additionally, it is cross-resistant to melphalan, adriamycin and irradiation, and these represent an excellent source for investigating tumor drug resistance.
They can replicate some of the physiological functions of ovarian cancer cells in in vitro culture (growth, division, metabolism, communication with the external world, ect.). This is an attribute that enables researchers to unravel the biology of ovarian cancer growth and development in detail in a relatively easy context. From examining gene expression, protein levels and cellular activity differences between drug-resistant and sensitive cell lines, it becomes possible to pinpoint specific molecules and signalling pathways responsible for drug resistance. In addition, this cell line could be utilized to test new anticancer drugs. A2780/cis cells received different potential anticancer drugs and inhibited cell growth, activated apoptosis, etc. were found to test these compounds on drug-resistant ovarian cancer cells. If a drug can effectively inhibit the proliferation of A2780/cis cells, it may become a new candidate for the treatment of drug-resistant ovarian cancer.
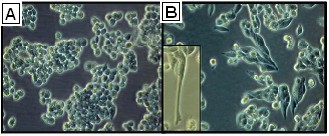 Fig. 1. Morphology of A2780 and A2780cis cells (Haslehurst AM, Koti M, et al., 2012).
Fig. 1. Morphology of A2780 and A2780cis cells (Haslehurst AM, Koti M, et al., 2012).
Auranofin and MC3 can Overcome Cisplatin Resistance Acting Independently from Cisplatin
Ovarian cancer, with the highest mortality among gynecological tumors, primarily due to late diagnosis, is predominantly treated with platinum-based drugs like cisplatin. Despite initial positive responses, resistance and recurrence pose significant challenges. Gold(I)-complexes, particularly N-heterocyclic carbene-gold(I) (NHC-Au(I)), have been researched as potential cytotoxic agents due to their unique mechanisms, notably inhibiting thioredoxin reductase (TrxR), thereby affecting redox balance and pathway regulation. König's team explores the effectiveness of gold(I) compounds auranofin and MC3 in overcoming cisplatin resistance in ovarian cancer cells.
A2780cis ovarian cancer cells exhibit approximately four times higher IC50 for cisplatin compared to A2780, indicating resistance (Fig. 1A, C). However, auranofin displays a higher cytotoxicity than cisplatin and partly overcomes the resistance in A2780cis cells (Fig. 1A). Notably, MC3 shows over tenfold higher activity than auranofin in both cell lines, indicating no resistance in A2780cis cells (Fig. 1C). In W1/W1CR cells, auranofin overcomes W1CR resistance (Fig. 1B), with MC3 again showing tenfold higher activity (Fig. 1D). To get a first insight into the probable mode of action of how Au(I)-compounds overcome cisplatin resistance in A2780cis and W1CR cells, a combinatory treatment was performed. Results reveal that pre-treatment with Au(I)-compounds does not affect cisplatin activity (Fig. 1E, F). Au(I)-drugs and cisplatin act neither similarly nor synergistically. They examined whether the high activity of auranofin and MC3 in cells is linked to high intracellular gold levels, potentially bypassing drug uptake or efflux resistance. Using AAS, they found that both auranofin (Fig. 1G) and MC3 (Fig. 1H) were present at only 25% or less in resistant cells compared to sensitive ones. This suggests that overcoming resistance in A2780cis and W1CR cells is not due to higher intracellular gold levels. Taken together, auranofin as well as MC3 are more cytotoxic than cisplatin and overcome cisplatin resistance in both cell lines not related to intracellular drug levels.
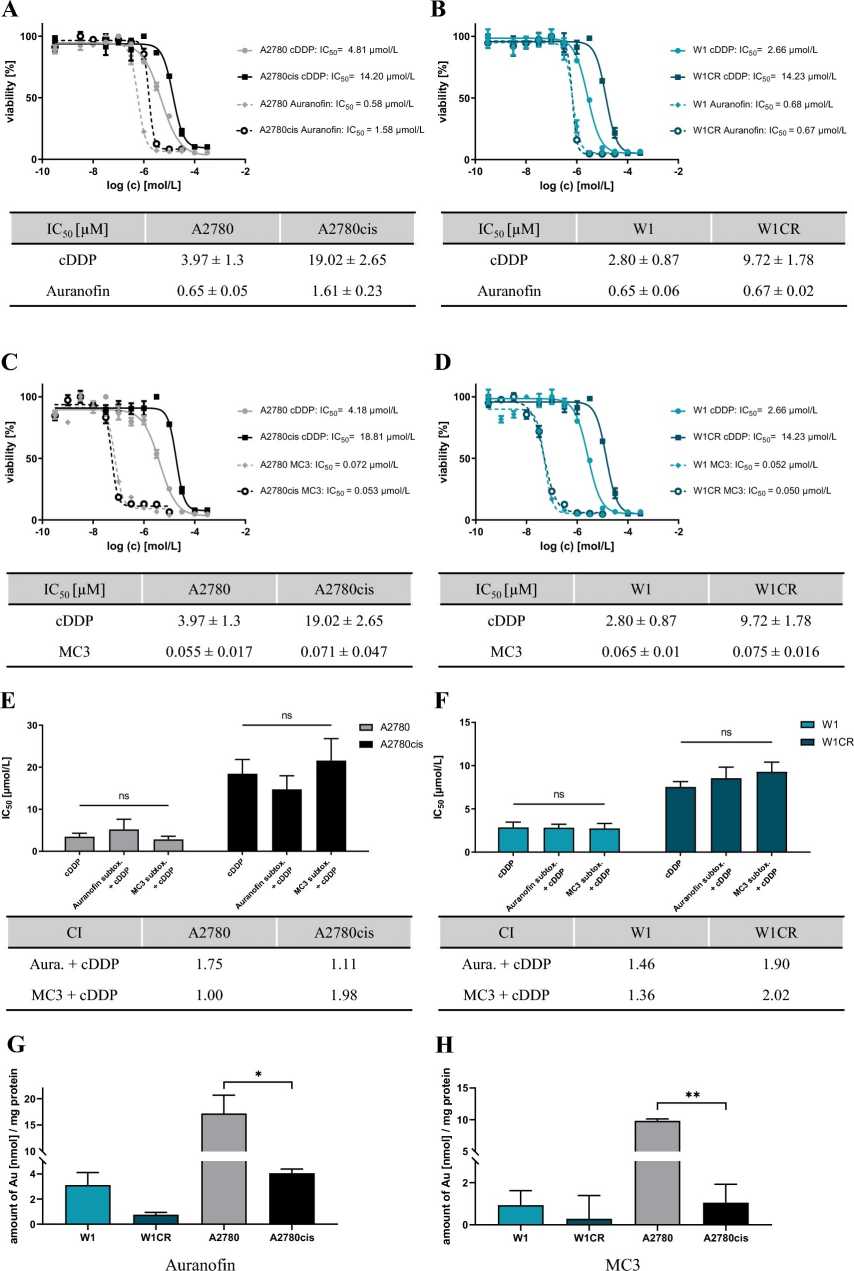 Fig. 1. Exemplary cytotoxicity curves of auranofin and MC3 compared to cisplatin (A-D). Histograms display the combinational treatment CI at IC50 (E-F). Intracellular gold levels in W1/CR and A2780/cis treated with auranofin (G) and MC3 (H) (König P, Zhulenko R, et al., 2023).
Fig. 1. Exemplary cytotoxicity curves of auranofin and MC3 compared to cisplatin (A-D). Histograms display the combinational treatment CI at IC50 (E-F). Intracellular gold levels in W1/CR and A2780/cis treated with auranofin (G) and MC3 (H) (König P, Zhulenko R, et al., 2023).
Anlotinib Inhibits the Proliferation of Cisplatin-Resistant OC Cells by Inducing the Expression of PLK2
Ovarian cancer is the most prevalent and lethal cancer among women. Though surgery and platinum-based chemotherapy remain standard treatment options, relapse rates are high due to resistance to drugs, particularly platinum. So new medications for platinum-resistant ovarian cancer are in high demand. Multi-target tyrosine kinase inhibitors (TKIs) such as anlotinib, which block multiple receptors such as VEGFR, have the potential to overcome resistance. But the therapeutic benefit of anlotinib for cisplatin-resistant (CIS) OC has not been described. Ji's team aimed to determine if anlotinib inhibited the pathogenesis of cisplatin-resistant OC.
Given anlotinib's anti-tumor activity on OC cells, they predicted that it would inhibit drug-resistant OC cell growth as well. Without treatment, cisplatin-resistant A2780 (A2780 CIS) cells grew more quickly than sensitive lines (Fig. 2A). Anlotinib inhibited A2780 CIS cell growth (Fig. 2B) and inhibited growth during colony-formation tests (Fig. 2C, D), resulting in G2/M arrest. They speculated anlotinib might also reduce migration and invasion in cisplatin-resistant OC cells. Wound-healing assays confirmed its effect, showing treated A2780 CIS cells migrated slower than controls (Fig. 2E, F) and had lower invasion ability (Fig. 2G, H). These results suggest anlotinib effectively inhibits proliferation, migration, and invasion in cisplatin-resistant OC cells. The downregulation of PLK2 is associated with ovarian cancer development and drug resistance. Using the GEO dataset GSE15372, PLK2 was identified as a differentially expressed gene linked to drug resistance in ovarian cancer (Fig. 3A). PLK2 expression was also reduced in drug-resistant ovarian cells as determined by Western blotting and qRT-PCR (Fig. 3B-D). This study found that anlotinib augmented PLK2 expression at the protein and mRNA levels in cisplatin-resistant ovarian cancer cells (Fig. 3E-G), which suggests that anlotinib might mitigate resistance by enhancing PLK2. To investigate this process, si-PLK2 cells were created. PLK2 knockdown was confirmed (Fig. 3H-J), accelerating cell growth. Anlotinib inhibited this growth, but the effect was weakened by PLK2 knockdown (Fig. 3K), indicating PLK2's role in anlotinib's anti-tumor effects.
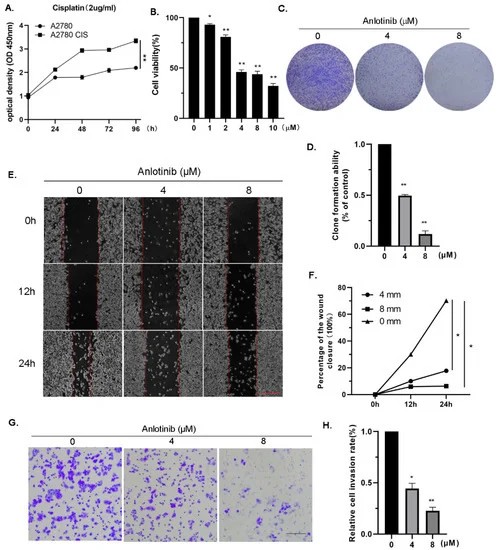 Fig. 2. Anlotinib inhibits the proliferation, migration, and invasion of cisplatin-resistant ovarian cancer cells in vitro (Ji Y, Li X, et al., 2022).
Fig. 2. Anlotinib inhibits the proliferation, migration, and invasion of cisplatin-resistant ovarian cancer cells in vitro (Ji Y, Li X, et al., 2022).
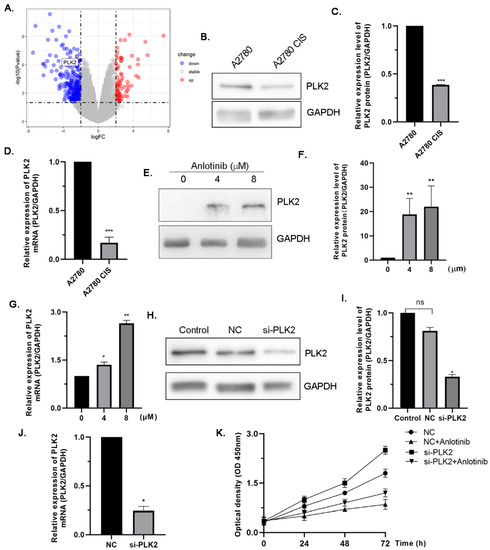 Figure 3. PLK2 is involved in mediating the antiproliferation effect of anlotinib (Ji Y, Li X, et al., 2022).
Figure 3. PLK2 is involved in mediating the antiproliferation effect of anlotinib (Ji Y, Li X, et al., 2022).
Ovarian cancer cells often exhibit characteristics such as uncontrolled growth, the ability to invade nearby tissues, resistance to cell death (apoptosis), and the potential to spread (metastasize) to other organs.
Ask a Question
Average Rating: 4.0 | 1 Scientist has reviewed this product
Good
The product was surprisingly frozen well, with minimal cell death observed during storage.
21 May 2023
Ease of use
After sales services
Value for money
Write your own review