Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
22Rv1 cells are a commonly used preclinical model of prostate cancer. Only recently, this cell line was classified as a biosafety level 2 cell line. This cell line produces high titers of xenotropic gammaretroviral particles which can infect human cells; inbred mice cells usually carry a mutation in the receptor of these viruses, called Xpr1, and are not permissive for this group of viruses. However, certain mouse cells (feral mice and some inbred strains carrying the appropriate receptor allele can be infected with the virus. Caution for the interpretation of data solely resulting from 22Rv1 cells carrying the virus has been discussed earlier however not directly addressed in in vivo or in vitro experiments.
22Rv1 Cells Expressing shLTR1+2 Differ in Cytokine Expression Pattern
22Rv1 cell line was shown to harbor multiple copies of a gammaretrovirus, called XMRV, integrated into its genome. While the original prostate cancer xenograft CWR22 is free of any retrovirus, subsequently generated cell lines 22Rv1 and CWR-R1, carry this virus and additionally shed infectious gammaretroviral particles in their supernatant. A 22Rv1 cell line was established with reduced XMRV transcript amounts. Stable hairpins containing the sequences shLTR1 and shLTR2 were individually cloned into the lentiviral vector LeGO G-puro.
Culture supernatants from 22Rv1 control cells, infected with pseudotyped lentiviral supernatant containing the empty LeGO G puro plasmid and from 22Rv1 shLTR1+2 cells were collected and assayed for cytokines by using a solid-phase immunoblotting procedure. The supernatants were applied to membranes containing antibodies to 80 human cytokines. Minor differences in the release of cytokines from these cells were detected. In 22Rv1 cells with reduced XMRV transcript levels, Osteopontin (OPN), a tissue inhibitor of matrix metalloproteinase TIMP2 and IL13 was slightly reduced while hepatocyte growth factor (HGF) was increased. Using quantitative real-time PCR for the indicated mRNA transcripts, these findings were confirmed: OPN, TIMP2, and IL13 expression is significantly reduced in 22Rv1 cells expressing shLTR1+2 while HGF expression is considerably increased (Fig. 1). Additionally, the expression of matrix metalloproteinase MMP9 and CXCL14 expression were tested in 22Rv1 cells and 22Rv1 shLTR1+2 cells. While no differences were found in MMP9 mRNA expression in these cell lines, CXCL14 expression was significantly reduced in the 22Rv1 shLTR1+2 cells.
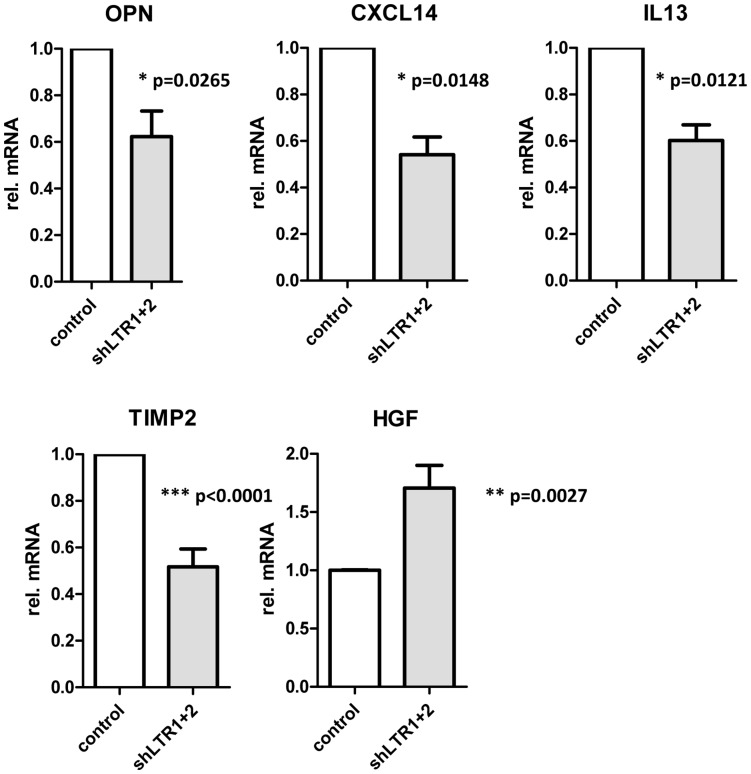 Fig. 1 Differences in cytokine expression pattern in 22Rv1 cells dependent on XMRV expression. (Stieler K, et al., 2012)
Fig. 1 Differences in cytokine expression pattern in 22Rv1 cells dependent on XMRV expression. (Stieler K, et al., 2012)
Growth Properties of PIV5 WT and Mutant Viruses in Prostate Cancer (22Rv1) and Benign (BPH-1) Cell Lines
The development of effective oncolytic viruses will require understanding the differences in virus replication and killing between normal and cancer cells. Infections of metastatic cancer (22Rv1) and benign non-tumorigenic (BPH-1) prostate cell lines were evaluated with a mutant parainfluenza virus 5 (P/V/F) encoding a defective V protein and a hyperfusogenic F protein. To determine the ability of PIV5 WT and mutant viruses to spread through a population of BPH-1 and 22Rv1, monolayers of cells were mock-infected or infected at a low multiplicity of infection (MOI) of 0.05 with PIV5 WT, P/V/F mutant or Le mutant. Bright-field (BF) and fluorescence images (FL) were taken 1, 2, and 3 days (D) post-infection (pi), and virus-infected cells were quantified by flow cytometry. As shown in Fig. 2A, WT, and mutant viruses showed a time-dependent spread through most of the 22Rv1 cell population by 3D pi. Quantification by flow cytometry showed that ~80% of infected 22Rv1 cells were GFP+ by 3D pi for all three viruses (Fig. 2C). By contrast, infected BPH-1 cells showed only low levels of GFP+ cells over time, with more WT and Le virus-infected cells positive at 3D pi compared to that of a P/V/F mutant (Fig. 2B). Flow cytometry quantification showed that ~20% of the BPH-1 cells were GFP+ when infected with either WT or Le mutant virus, compared to only ~10% of the cells infected with the P/V/F mutant by 4D pi (Fig. 2D).
Multi-step growth properties of WT and mutant viruses in 22Rv1 and BPH-1 cells were determined by infecting with control WT virus at a MOI of 0.01, while P/V/F and Le mutants infections were carried out at a MOI of 0.05. Media from infected cells were harvested at indicated time points shown in Fig. 2E, F, and virus titers were determined by plaque assay. In 22Rv1 cancer cells, growth of the P/V/F and Le mutant viruses was to slightly higher titers than PIV5 WT, with the P/V/F mutant growing to ~2 logs higher in titer than the other viruses by 4D pi. By contrast, in BPH-1 benign cells, growth of the P/V/F mutant peaked at a titer slightly higher than 103 PFU/mL by 2D pi but then was reduced to the limit of detection at later time points (Fig. 2F). PIV5 WT and Le mutant grew to similar titers, which were 103 fold higher than the P/V/F mutant. All viral titers from BPH-1 cells across the time course were lower compared to corresponding titers observed in the 22Rv1 cells (Fig. 2E). Taken together, these data indicate that the WT and mutant viruses are capable of comparable growth in and spread through a population of 22Rv1 cells, but the P/V/F mutant is restricted in growth in BPH-1 cells relative to WT or Le mutant viruses.
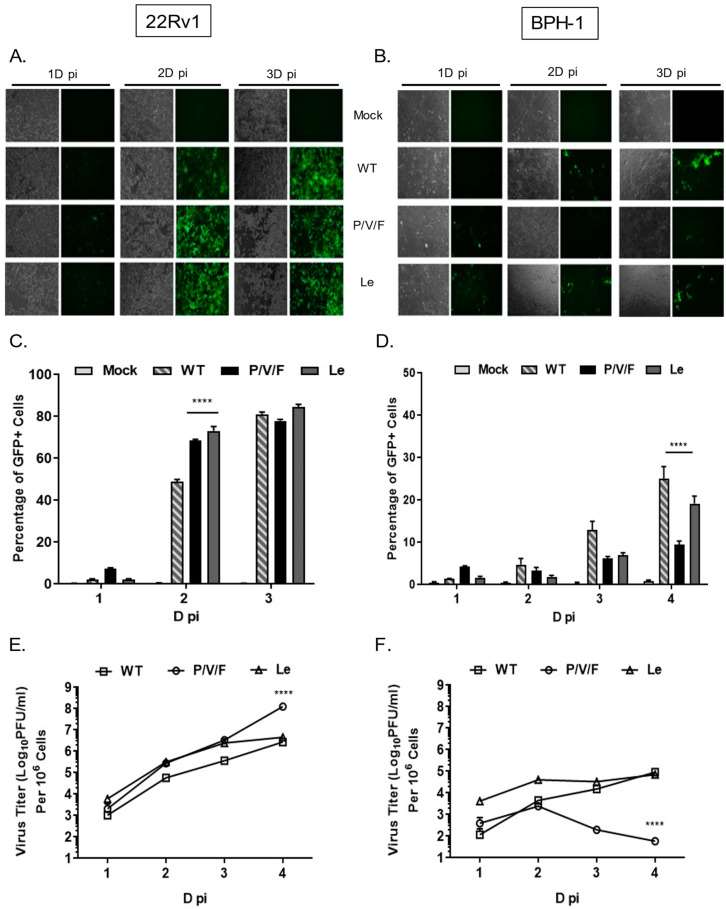 Fig. 2 Spread and growth of PIV5 WT and mutant viruses in prostate cancer and benign cells. 22Rv1 (A, C, E) and BPH-1 cells (B, D, F) were either mock-infected or infected with the control WT virus or the indicated mutant PIV5 viruses at a MOI of 0.05. (Kedarinath K, et al., 2022)
Fig. 2 Spread and growth of PIV5 WT and mutant viruses in prostate cancer and benign cells. 22Rv1 (A, C, E) and BPH-1 cells (B, D, F) were either mock-infected or infected with the control WT virus or the indicated mutant PIV5 viruses at a MOI of 0.05. (Kedarinath K, et al., 2022)
h.i. FBS cells display features that resemble prostate stromal cells found in the human body, including cell shape, gene expression, and certain functional characteristics.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
Highly recommend
The customer service team at Creative Bioarray was excellent, providing helpful guidance and support throughout. I highly recommend Creative Bioarray for anyone in need of high-quality tumor cells for scientific research purposes.
09 May 2023
Ease of use
After sales services
Value for money
Write your own review
- You May Also Need