Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
BPH-1
Cat.No.: CSC-C0299
Morphology: adherent epitheloid cells growing as monolayers
- Specification
- Background
- Scientific Data
- Publications
- Q & A
- Customer Review
The prostate is the major reproductive gland and affects male fertility and health. Microscopically, the prostate gland mainly consists of stromal and epithelium cells. The growth and development of the prostate are induced by stromal-helix interactions. The benign prostatic hyperplasia epithelial cell line (BPH-1) originated from human prostate tissue after transurethral resection and was first isolated and identified by S.W. Hayward et al. in 1994. The BPH-1 cell line lacks the expression of the androgen receptor. Uncontrolled proliferation of BPH-1 cells can induce BPH and lead to bothersome complications, such as hematuria and renal insufficiency, significantly impairing a patient's quality of life. BPH-1 cells stimulate aromatase expression in prostatic stromal cells (PrSCs) by producing prostaglandin E2 in a paracrine manner. Aromatase can convert testosterone into estradiol and have high expression in BPH patients. As a well-established model for the human prostate biology of BPH, the BPH-1 cell line has been exploited to research anti-BPH candidates.
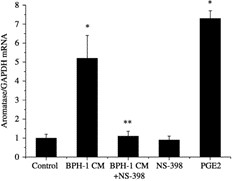 Fig. 1 Real-time RT-PCR analysis of aromatase mRNA expression in prostatic stromal cells treated with BPH-1 conditioned medium with or without NS-398 (10−6 mol/l) and PGE2 (10−7 mol/l). (Jin BR, et al., 2024)
Fig. 1 Real-time RT-PCR analysis of aromatase mRNA expression in prostatic stromal cells treated with BPH-1 conditioned medium with or without NS-398 (10−6 mol/l) and PGE2 (10−7 mol/l). (Jin BR, et al., 2024)
Apo Exerts Anti-proliferative, Anti-oxidant, and Anti-inflammatory Effects in BPH-1 Cells
Apocynin (Apo), an NADPH oxidase (NOX) inhibitor, has been widely used to treat various inflammatory diseases. To investigate the pharmacological effects of Apo on prostate cancer cells, an in vitro BPH model was established using BPH-1 cells. Using the CCK-8 assay, the effective dose of Apo was confirmed to suppress the proliferation of BPH-1 cells was > 7.81 μM (Fig. 2A). To investigate whether Apo exerts anti-oxidant activity in BPH-1 cells, an immunofluorescence assay was performed to detect 8-OHdG, a biomarker of oxidative stress. BPH-1 cells exhibited green fluorescence in the nucleus and cytosol, indicating oxidative stress. Treatment with 500 μM Apo mitigated the enhanced green fluorescence observed in the BPH-1 cells. In contrast to the untreated group, the group stimulated with exogenous H2O2 exhibited markedly increased green fluorescence intensity. Treatment with Apo suppressed H2O2-induced upregulation of 8-OHdG fluorescence intensity (Fig. 2B). In addition, Apo significantly upregulated the levels of the anti-oxidant genes HMOX1, SOD1, and GPX1 (Fig. 2C). Treatment with Apo also significantly downregulated NOS2, PTGS2, IL6, and TNFA levels in BPH-1 cells (Fig. 2D). Furthermore, Apo significantly upregulated the levels of the M2 macrophage markers MRC1 and IL10 (Fig. 2E) and suppressed the upregulated levels of the M1 macrophage markers CD68, CD80, and CD86 in BPH-1 cells (Fig. 2F). NOX4 is a major source of oxidative stress and has pro-inflammatory actions. As shown in Fig. 2G and 2H, treatment with Apo significantly suppressed the expression of NOX4. Thus, Apo exerts anti-proliferative, anti-oxidant, and anti-inflammatory effects on BPH-1 cells via inhibition of NOX4 expression.
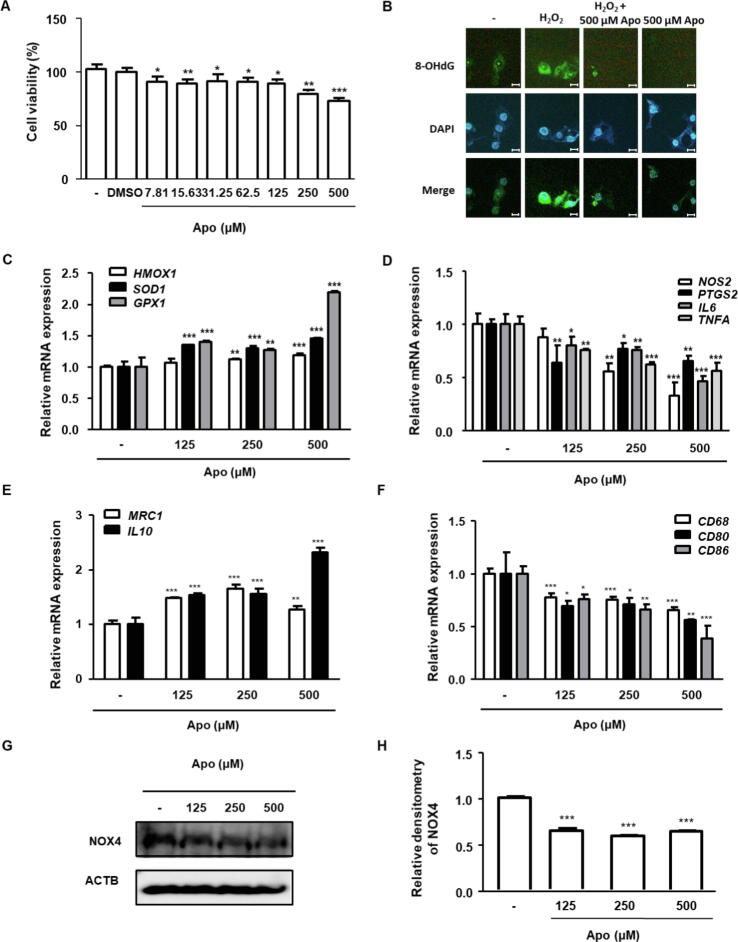 Fig. 2 (A) Effect of Apo on the viability of BPH-1 cells (B) Effect of Apo on oxidative stress in BPH-1 cells. (C-F) BPH-1 cells were treated without or with Apo (125, 250, or 500 μM). (G) The protein level of NOX4 was determined. (H) The levels of target protein were normalized to that of ACTB (internal control) and are presented as relative protein levels. (Jin BR, et al., 2024)
Fig. 2 (A) Effect of Apo on the viability of BPH-1 cells (B) Effect of Apo on oxidative stress in BPH-1 cells. (C-F) BPH-1 cells were treated without or with Apo (125, 250, or 500 μM). (G) The protein level of NOX4 was determined. (H) The levels of target protein were normalized to that of ACTB (internal control) and are presented as relative protein levels. (Jin BR, et al., 2024)
Effects of Cu B on BPH-1 Cell Viability and Apoptosis
Cucurbitacin B (Cu B), a triterpenoid compound, has anti-inflammatory and antioxidant activities. The effects of Cu B on BPH-1 cells were evaluated using a CCK-8 assay. As Fig. 3 shows, Cu B (12.5-200 nM) inhibited the growth of BPH-1 cells. Meanwhile, doxazosin was selected as the positive control to evaluate the antiproliferative activity difference between Cu B and doxazosin on prostate cells. After treating cells with Cu B for 48 h, the inhibitory effect of Cu B (50-200 nM) on BPH-1 cell proliferation was slightly stronger than that of doxazosin (40 μM). Moreover, cytomorphological observation indicated that compared with the control group, Cu B treatment induced distinct morphological alterations, manifesting as cell shrinkage, rounding, and karyorrhexis (Fig. 4). To further evaluate the efficacy of Cu B on BPH-1 cells, Cu B-treated cell apoptosis rates were measured using flow cytometry. Annexin V-FITC/PI staining highlighted that after treatment with Cu B and Doxa, the apoptosis rates of BPH-1 cells (Fig. 5) were drastically increased. The apoptosis index of BPH-1 cells in the high-dose Cu B group (50 nM and 100 nM) was remarkably higher than that in the control group.
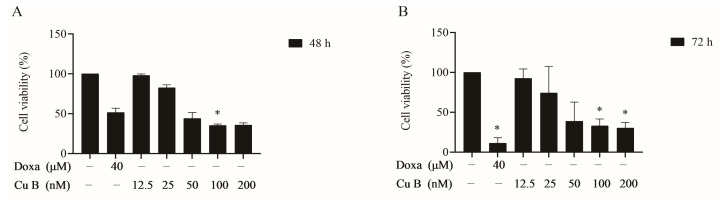 Fig. 3 Effect of Cu B on the cell viability of BPH-1 cells based on CCK-8 results. (Zhou P, et al., 2023)
Fig. 3 Effect of Cu B on the cell viability of BPH-1 cells based on CCK-8 results. (Zhou P, et al., 2023)
 Fig. 4 Effect of Cu B (50-200 nM) and doxazosin (40 μM) on the cellular morphology of BPH-1 cells. (Zhou P, et al., 2023)
Fig. 4 Effect of Cu B (50-200 nM) and doxazosin (40 μM) on the cellular morphology of BPH-1 cells. (Zhou P, et al., 2023)
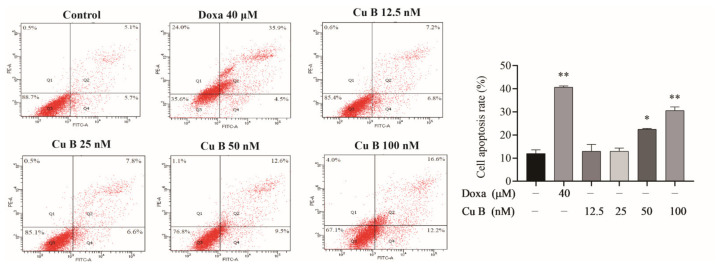 Fig. 5 Cu B and Doxa induced the apoptosis of BPH-1 cells based on the results of the flow cytometry analysis. (Zhou P, et al., 2023)
Fig. 5 Cu B and Doxa induced the apoptosis of BPH-1 cells based on the results of the flow cytometry analysis. (Zhou P, et al., 2023)
Scientists use h.i. FBS cells to investigate the impact of various factors, such as hormones or drugs, on prostate tumor growth, metastasis, and response to treatment.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
Satisfied
I purchased prostate tumor cells from Creative Bioarray for my scientific research, and I am extremely satisfied with both the quality of the cells and the service I received.
23 Jan 2023
Ease of use
After sales services
Value for money
Write your own review
- You May Also Need