What are the Pharmacokinetic Properties of the Antisense Oligonucleotides?
Oligonucleotide therapeutics are nucleic acid drugs that function therapeutically by binding to RNA or DNA molecules, thus suppressing gene expression or modifying gene activity.
Classification and Characteristics of Oligonucleotide Drugs
The most common oligonucleotide therapies are antisense oligonucleotides (ASOs), small interfering RNAs (siRNAs), ribozymes, deoxyribozymes, antigene products, transcription factor decoys, and aptamers. Synthetic oligonucleotides with chemical modifications have greater stability and can modulate gene expression via RNA interference, target degradation by RNase, splicing modulation, non-coding RNA inhibition, gene stimulation and programmable gene editing. Such technologies are at the cutting edge of emerging drug discovery. The majority of therapeutically approved oligonucleotides are RNA-based treatments, such as ASOs and siRNAs. Since ASOs were identified in 1978, 12 ASO drugs have been approved, accounting for 60% of oligonucleotide drugs. These activities of ASOs can alter the production or activity of disease-causing proteins through changes in pre-mRNA splicing, mRNA degradation, protein translation, or direct interactions with proteins.
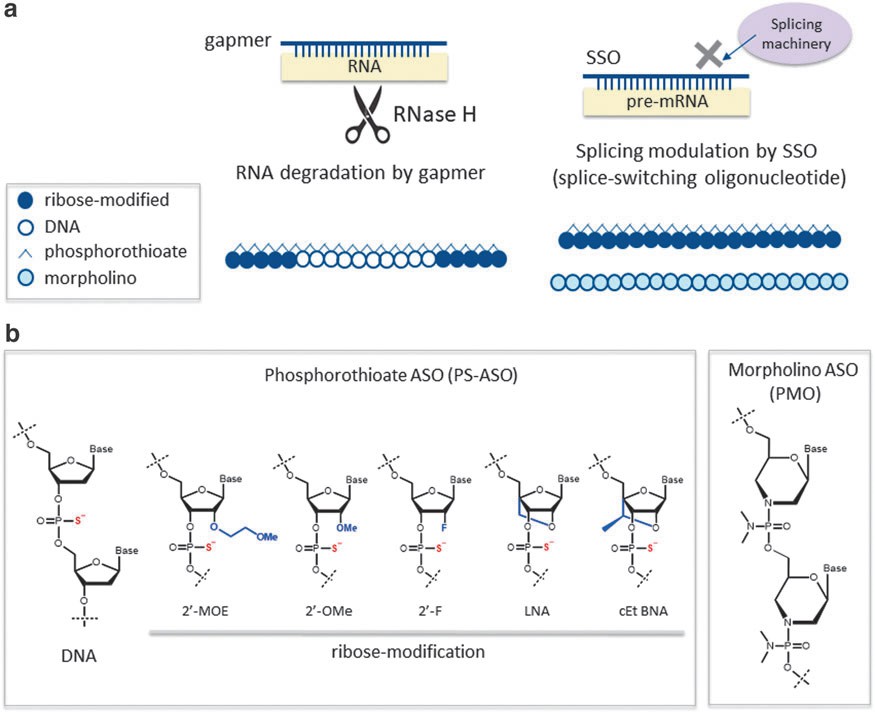 Fig. 1. Classification and structures of ASO therapeutics (Takakusa H., Iwazaki N., et al., 2023).
Fig. 1. Classification and structures of ASO therapeutics (Takakusa H., Iwazaki N., et al., 2023).
As ASOs can directly change the production of pathogenic proteins, there is considerable promise that they will offer novel treatments for a wide range of human conditions. While current evaluation strategies tend to draw on techniques that have been used for small-molecule medications, ASOs have distinctive drug metabolism and pharmacokinetic (DMPK) features. These following sections will briefly describe the DMPK of ASOs.
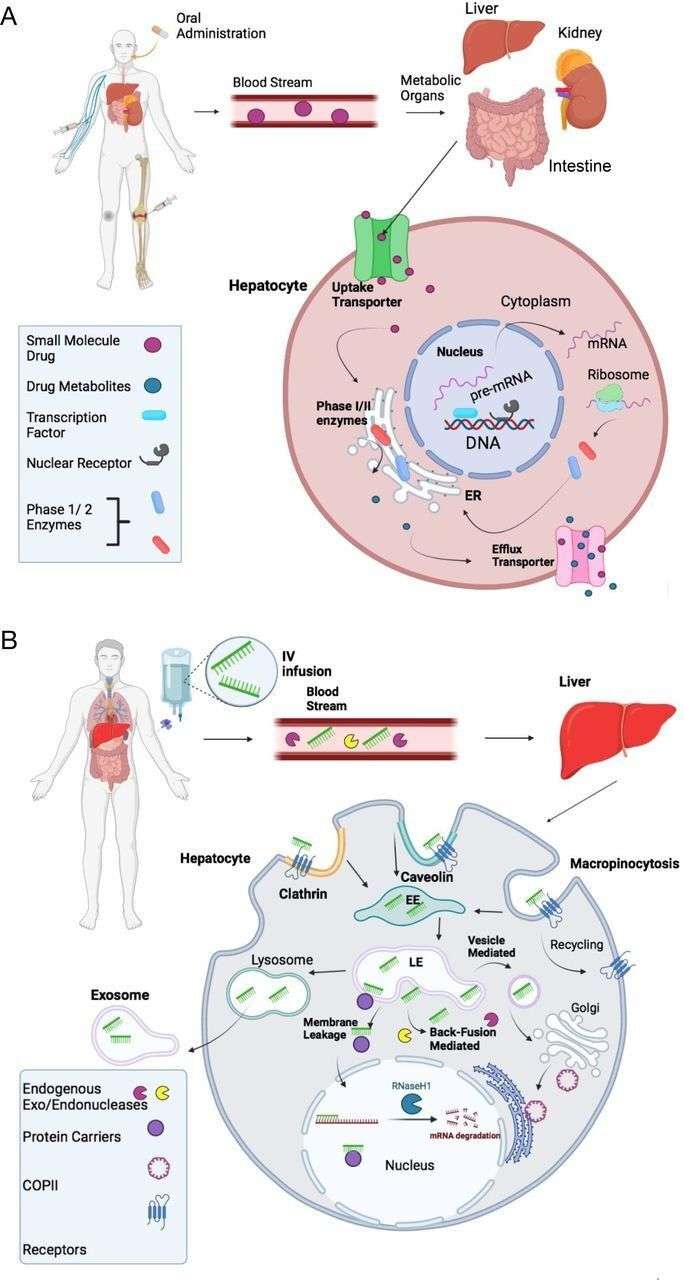 Fig. 2. Comparison of absorption, distribution, metabolism, and excretion (ADME) of small chemical drugs and ASO drugs (Migliorati JM., Liu S., et al., 2022).
Fig. 2. Comparison of absorption, distribution, metabolism, and excretion (ADME) of small chemical drugs and ASO drugs (Migliorati JM., Liu S., et al., 2022).
Absorption and Distribution of Oligonucleotide Drugs
ASOs are typically administered via local injections (e.g, intravitreal or intrathecal), subcutaneous (SC) and intravenous (IV) injections. Systemically administered oligonucleotide therapies tend to spread preferentially among tissues with discontinuous or fenestrated capillaries (the liver, kidneys), and poor in the central nervous system, eyes, placenta, and other tissues with almost continuous capillaries.
The absorption efficiency of ASOs depends on solubility, membrane permeability, chemical composition, formulation, location, and route of administration. These include ASO-protein interactions; their specificity to proteins is dependent on PS, gapmer design, and sequence. For instance, PS-ASOs with negatively polarised backbones show an exceptional affinity for plasma proteins, especially albumin (> 85%). Neutral PMOs, on the other hand, do not bind as well to plasma proteins, which causes a 10-fold difference in ASO-protein affinity. The presence of PS connections raises the plasma half-life to 1-2 hours. After 12 hours, the condition settles to an unreactive state, with less than 1% of the ASO left in the plasma. The chemistry of the 2′-position with moieties like methoxyethyl (MOE), constrained ethyl (cEt), or locked nucleic acid (LNA) shuts down exonuclease and prolongs the half-life of tissue elimination to up to four weeks.
After being absorbed, ASOs diffuse into the systemic circulation and irreversibly associate with proteins and lipids in plasma and tissues. Only a small portion of the drug not eliminated by macrophages, through endocytosis, is released into the cytoplasm or nucleus, where it binds to mRNA and acts pharmacologically. Intracellular ASOs may have longer half-lives (1-4 weeks) and prolonged biological activity. Importantly, the mean of all tissues could be underestimated because oligonucleotides can either get to non-parenchymal cells (i.e., sinusoidal endothelial cells and Kupffer cells in the liver) or become trapped inside the lysosomal compartments of parenchymal cells.
Table. 1. Local and systematic absorption of the FDA-approved ASO drugs (Migliorati JM., Liu S., et al., 2022).
| Drug | AdministrationSite and Route | Local vs. Systematic Absorption | Ability to Cross Blood-Brain Barrier | Tissues with Highest Drug Concentrations | Peak Plasma Concentration Time (Hours) |
| Fomivirsen | Intravitreal injection | Local eye | No | Retina, iris; systemic exposure: minimal (below limits of quantitation) | Very low |
| Nusinersen | Intravitreal injection | CNS and systematic | Yes | CNS, plasma, peripheral tissue (skeletal muscle, liver, kidney), fat, bone marrow, spleen | 1.7-6 |
| Pegaptanib | Intravitreal injection | Local eye | No | Vitreous fluid, retina, aqueous fluid; systemic exposure: kidney | Slowly absorbed into systemic circulation |
| Mipomersen | S.C. injection | Systematic | No | Liver, kidney, bone marrow, adipose tissue, lymph nodes | 3-4 |
| Inotersen | S.C. injection | Systematic | No | Liver, kidney, broad tissues | 2-4 |
| Defibrotide | I.V. infusion | Systematic | No | Cells lining blood vessel in liver | 2 |
| Eteplirsen | I.V. infusion | Systematic | No | Kidney, skeletal muscles | 1-2 |
| Golodirsen | I.V. infusion | Systematic | No | Kidney, all tissue except CNS | 1 |
| Viltolarsen | I.V. infusion | Systematic | No | Kidney, skeletal muscle | 1 |
| Casimersen | I.V. infusion | Systematic | No | Kidney, skeletal muscle | 1 |
Table. 2. Biodistribution of the FDA-approved ASO drugs (Migliorati JM., Liu S., et al., 2022).
| Drug | Biodistribution | |||
| Protein Binding | Bioavailability | AUC | Volume of Distribution | |
| Fomivirsen | 40% (from analysis of vitreous samples from treated rabbits and monkey) | N/A | N/A | N/A |
| Nusinersen | CSF:<25%; plasma:="">94% | 100% (intrathecal) | N/A | CSF: 0.4 L; plasma: 29 L |
| Pegaptanib | N/A | N/A | 25 lg/h per ml (at 3 mg monocular dose) | N/A |
| Mipomersen | >90% at clinically relevant concentrations (1–8 lg/ml) | 54% to 78% | N/A | N/A |
| Inotersen | >94% (independent of dosage) | N/A | 90 mcg/h per ml | 293 L |
| Defibrotide | Average of 93% | 100% (i.v.) | 26.9–48.1 lg/ml × h | 8.1–9.1 L |
| Eteplirsen | 6% to 17% | 100% (i.v.) | N/A | 600 ml/kg |
| Golodirsen | 33% to 39% (independent of dosage) | 100% (i.v.) | 34% to 44%a | 668 ml/kg (at dose of 30 mg/kg) |
| Viltolarsen | Average of 40% | 100% (i.v.) | 16% to 27%a | 300 ml/kg |
| Casimersen | 8% to 32% | 100% (i.v.) | 16% to 34%a | 367 ml/kg |
Metabolism of Oligonucleotide Drugs
ASOs are typically metabolised by nucleases and their metabolism stability varies significantly between species and molecules, particularly for their chemical structure and sugar modifications. Exonucleases usually attack the outer ends of ASOs; endonucleases attack the inside. Long-lived and short-lived ASOs are discharged into the urine via membrane leakage, vesicle release or exocytosis. Metabolites are created rather slowly and are excreted relatively quickly - reducing plasma exposure. In contrast to small molecules, which are broken down by CYP450 enzymes, there are no well-developed in vitro metabolic experiments or techniques with oligonucleotides. But for the phosphorothioate ASOs (PS-ASOs), there are common pathways in several compounds found in mice, monkeys, rats and humans, so there is no species-specific difference in the metabolism of PS-ASOs.
Elimination of Oligonucleotide Drugs
Due to their charge, polarity and hydrophilicity, ASOs are mostly eliminated in urine or faeces as unbroken molecules or as metabolites reduced by nuclease activity. Excessive excretion will vary depending on the chemical structure of the ASO, the route of administration, species, and conjugates. The amount of protein binding also makes a difference in the process of excretion. Some ASOs, like phosphorothioate-modified antisense oligonucleotides (PS-ASOs), are usually less excreted, which means longer time to observe.
Table. 3. Pharmacokinetics of ASOs Dependent on Administration Route (Kim Y., 2023).
| Route | Absorption | Distribution | Metabolization | Elimination |
| Intravenous | zero-order input/direct (blood) | blood plasma/vessels, surrounding tissueand organs (liver, bile, heart, lung, kidney), high bioavailability | exonucleolytic, primarily in liver, blood plasma, various cell types, dependent on applied ASO chemistry | very soon, multiphasic kinetics, long terminal half-life, renal, feces, only metabolites |
| Intra-peritoneal | delayed, peritoneal blood vessels, gastrointestinal | blood plasma/vessels, surrounding tissue and organs (intestine, ascites fluid, liver, bile, heart, lung, kidney), bioavailability comparable to i.v. | exonucleolytic, gastrointestinal, degradation, in liver (via portal vein, first pass effect), various cell types, dependent on applied ASO chemistry | late, renal, feces, only metabolites |
| Intra-cardiac | zero-order input/direct (heart) | blood plasma/vessels, organs (heart, lung, liver, bile, kidney), high bioavailability | exonucleolytic, primarily in liver, blood plasma, various cell types, dependent on applied ASO chemistry | exonucleolytic, primarily in liver, blood plasma, various cell types, dependent on applied ASO chemistry |
| Intratumoral or Intrathecal | tumor, blood plasma/ vessel | blood plasma/vessels, surrounding tissue and organs (liver, bile, heart, lung, kidney) | exonucleolytic, in liver, blood plasma, various cell types, dependent on applied ASO chemistry | fast from tumor into blood plasma |
| Subcutaneous | delayed, fat, reservoir effect | blood plasma/vessels (delayed), surrounding tissue, organs | exonucleolytic, in blood plasma, various cell types, dependent on applied ASO chemistry | exonucleolytic, in blood plasma, various cell types, dependent on applied ASO chemistry |
| Intramuscular | delayed, muscle, reservoir effect | muscle, blood plasma/vessels (delayed), surrounding tissue, organs | exonucleolytic, in blood plasma, various cell types, dependent on applied ASO chemistry | from blood plasma, multiphasic kinetics, long terminal half-life |
Future Perspectives
The exploration of new ASO therapies is taking off fast, and there are more ASOs than ever being used to treat genetic disorders and other rare diseases. Modern drug metabolism and pharmacodynamics (DMPK) research contributes not only to the drug design and dosage but also to drug targeting and side-effect reduction. This is why understanding the DMPK of ASOs is crucial for their development and safety.
Creative Bioarray Relevant Recommendations
| Products & Services | Description |
| In Vitro DMPK Services | Creative Bioarray provides a variety of in vitro ADME/PK services, including high-throughput ADME screening, in vitro binding, in vitro metabolism, in vitro permeability, and transporter assays. |
| In Vitro DMPK Services | Creative Bioarray provides a variety of in vitro ADME/PK services, including high-throughput ADME screening, in vitro binding, in vitro metabolism, in vitro permeability, and transporter assays. |
References
- Geary RS, et al. Clinical and preclinical pharmacokinetics and pharmacodynamics of mipomersen (Kynamro®): a second-generation antisense oligonucleotide inhibitor of apolipoprotein B." Clin Pharmacokinet, 2015, 54 (2), 133–146.
- Takakusa H, et al. Drug Metabolism and Pharmacokinetics of Antisense Oligonucleotide Therapeutics: Typical Profiles, Evaluation Approaches, and Points to Consider Compared with Small Molecule Drugs. Nucleic Acid Ther, 2023, 33 (2), 83-94
- Migliorati JM, et al. Absorption, Distribution, Metabolism, and Excretion of US Food and Drug Administration-Approved Antisense Oligonucleotide Drugs. Drug Metab Dispos, 2022, 50(6):888-897.
- Shadid, M., et al. Antisense oligonucleotides: absorption, distribution, metabolism, and excretion. Expert opinion on drug metabolism & toxicology, 2021, 17 (11), 1281–1292.
- Bäckström E, et al. Tissue pharmacokinetics of antisense oligonucleotides. Mol Ther Nucleic Acids, 2024, 35 (1), 102133.
- Kim Y. Drug Discovery Perspectives of Antisense Oligonucleotides. Biomol Ther (Seoul), 2023, 31 (3), 241-252.

