ISH probe labeling method
In Situ is an essential molecular biology method for detecting and localising nucleic acid sequences. The technique enables spatially specific localisation by hybridising labeled probes with sequences of nucleic acids in a sample. In this article, we explore probe markers, different benefits and drawbacks, uses, and considerations when choosing the best approach.
Types of probe labeling
Radioactive isotope labeling:
Initial ISH methodologies employed radioactive isotope labeling (using 32P or 35S isotopes). This technique is highly sensitive, but it is rarely applied to laboratories today due to the risks associated with radioactive exposure.
Fluorescent labeling:
This technique employs fluorescent molecules (such as Fluorescein, Cy3, Cy5) attached to the probe. Under a fluorescent microscope, hybridisation signals are visible. Fluorescent labeling is sensitive, versatile, allows for multicolor detection, and is technically straightforward, making it the preferred method for fluorescence in situ hybridization (FISH). Today, FISH technology supports automation and high-throughput detection for large sample volumes processing. However, fluorescent labeling can suffer from photobleaching, where the fluorescent signal diminishes over time. This limitation can affect the long-term visualization of samples.
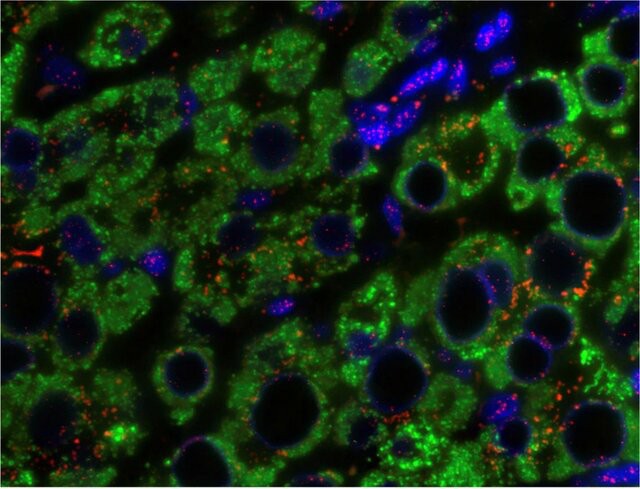 Fig. 1. Q-FISH of liver tissue in chronic HBV infection (Tachtatzis, P. M., Marshall, A., et al., 2015).
Fig. 1. Q-FISH of liver tissue in chronic HBV infection (Tachtatzis, P. M., Marshall, A., et al., 2015).
Biotin labeling:
Biotin, a small molecule, can be chemically bound to the probe. Post-hybridization, the biotin-labeled probe hybridized to target sequences is detected using proteins like Streptavidin or Avidin, which specifically bind to biotin. This method offers high sensitivity and specificity but requires caution due to potential interference from endogenous biotin.
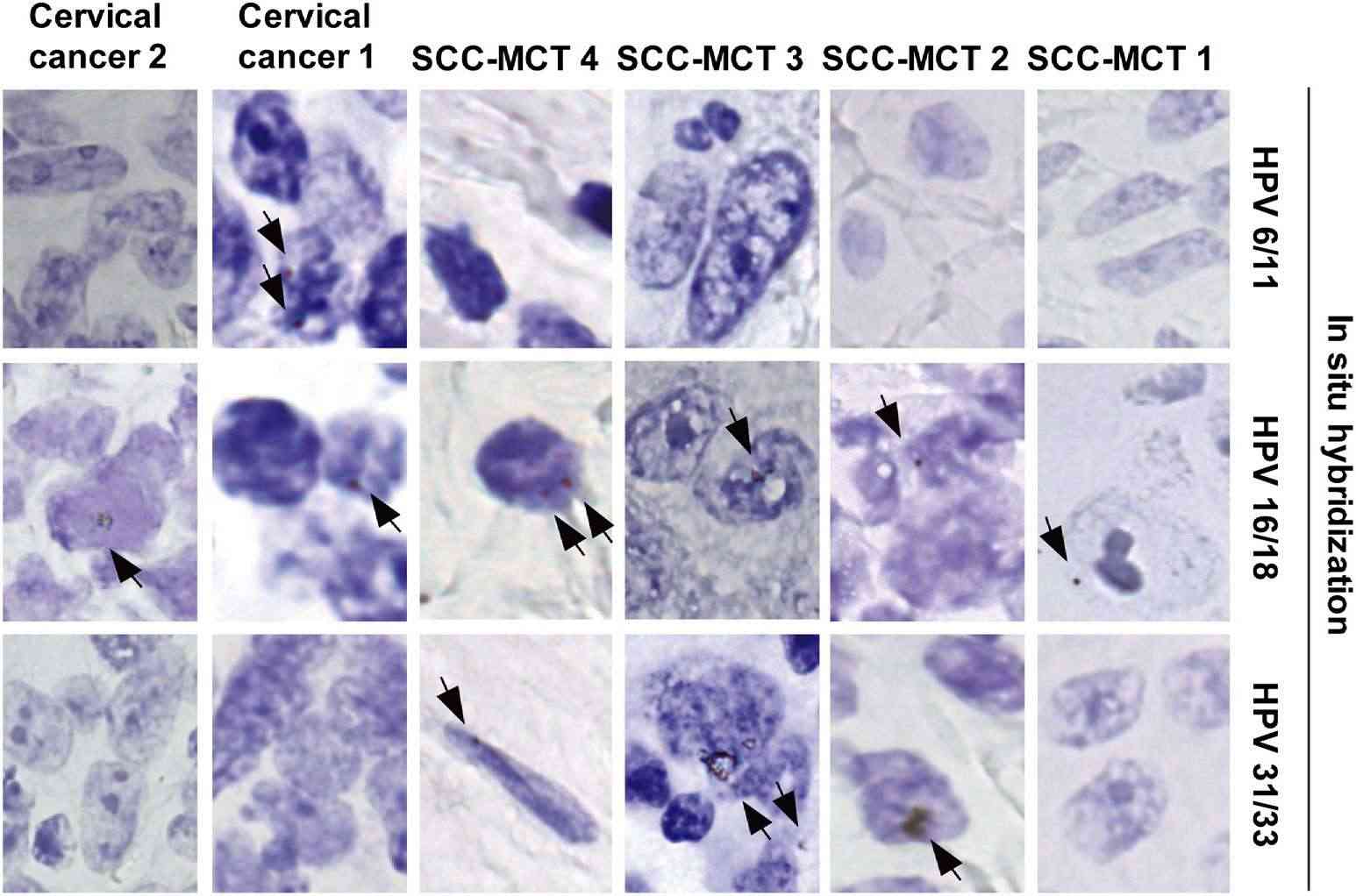 Fig. 2. Human papillomavirus (HPV) detection by in situ hybridization (biotin-labeled DNA probes) (Chiang, A. J., Chen, D. R., et al., 2015).
Fig. 2. Human papillomavirus (HPV) detection by in situ hybridization (biotin-labeled DNA probes) (Chiang, A. J., Chen, D. R., et al., 2015).
Digoxigenin (DIG) labeling:
DIG, a plant-derived steroid compound, works similarly to biotin but presents a lower endogenous background. DIG-labeled probes are detected with anti-DIG antibodies. This technique maintains high sensitivity and specificity with reduced background interference.
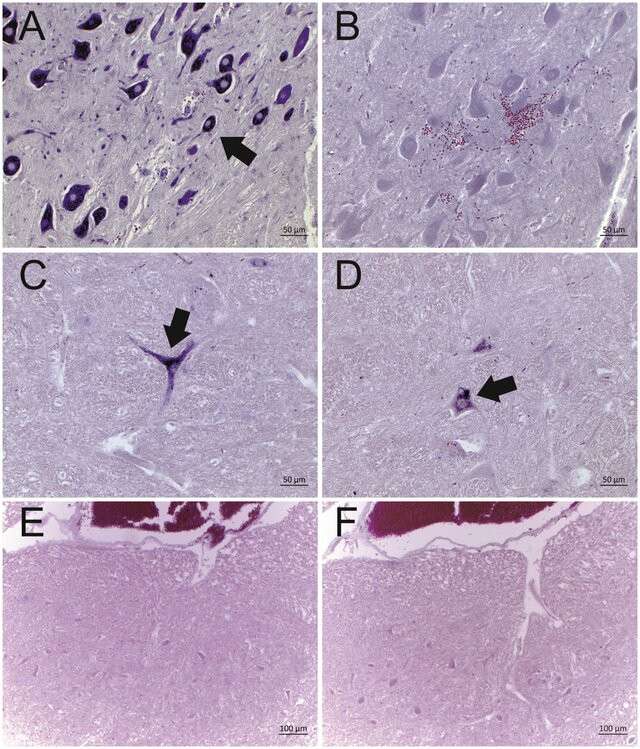 Fig. 3. Representative results of the in situ hybridization for BoAstV-CH13/NeuroS1 RNA in cattle brain tissues (DIG labeled RNA probes) (Selimovic-Hamza, S., Bouzalas, I. G.,et al. 2016).
Fig. 3. Representative results of the in situ hybridization for BoAstV-CH13/NeuroS1 RNA in cattle brain tissues (DIG labeled RNA probes) (Selimovic-Hamza, S., Bouzalas, I. G.,et al. 2016).
Chemiluminescent labeling:
This method uses chemiluminescent substances (e.g., Horseradish Peroxidase [HRP], Alkaline Phosphatase) to label probes. Detection is facilitated through substrate-induced chemiluminescent emission. While it offers high sensitivity, the procedure can be relatively complex. Because this method requires precise substrate handling and timing to capture the luminescent signal accurately. Background signal interference can obscure results if not optimally managed. Chemiluminescent labeling has been successful in gene expression studies, particularly for identifying the overexpression of oncogenes in tumor tissues, allowing researchers to achieve highly sensitive detection of mRNA levels.
Nanoparticle labeling:
Nanotechnology has also led to the application of nanoparticles like Quantum Dots and Gold Nanoparticles as probe labels. These nanoparticles are utilized based on their inherent optical properties, giving great optical stability and multi-color ability at a higher cost. Nanoparticle synthesis and operation are also technically challenging, limiting their widespread use.
Criteria for Choosing a Probe Labeling Method
- Target abundance: For samples with low target abundance, highly sensitive labeling methods such as fluorescent labeling, nanoparticle labeling, or biotin labeling combined with signal amplification techniques are recommended. Biotin-labeled probes can be amplified using enzyme conjugates like Streptavidin-HRP to enhance detection sensitivity. For multiplex detection, fluorescent labeling is ideal due to its ability to visualize different targets using distinct fluorescent colors.
- Sample type: Different sample types may require different labeling methods. For example, cell samples are well-suited for fluorescent labeling as fluorescent microscopy can clearly visualize hybridization signals within cells. Tissue sections might necessitate consideration of probe penetration and background interference. Biotin labeling is highly sensitive but may face interference from endogenous biotin, while DIG labeling often provides lower background levels.
- Ease of operation: Methods such as fluorescent labeling and DIG labeling are user-friendly, involve fewer steps, and are easy to master. For instance, FISH can be used for accurate cytogenetic diagnostic applications, including checking for chromosomal abnormalities using FISH in prenatal diagnostics. The procedure is fast and precise which makes it a valuable option in clinical cases with high turnaround times. Radioactive isotope labeling, on the other hand, involves storing and transporting radioactive substances, which are harder to do and pose hazards.
- Equipment and reagent availability: The choice of labeling method also depends on the laboratory's equipment and reagent availability. For example, fluorescent labeling necessitates a fluorescent microscope, while DIG labeling requires specific antibodies and substrates for detection.
By considering these factors comprehensively, researchers can select the most suitable ISH probe labeling method to meet the specific requirements of their experimental protocols.
| Products & Services | Description |
| Fluorescent In Situ Hbridization (FISH) Service | Creative Bioarray offers a range of different FISH services including metaphase and interphase FISH, fibre-FISH, RNA-FISH, M-FISH, 3D-FISH, flow-FISH, FISH on paraffin sections, and immune-FISH. |
| FISH Probe Design, Synthesis, and Testing Service | Creative Bioarray is capable of developing custom FISH probes. Apart from that, we also offer mRNA ISH/FISH probes, miRNA ISH/FISH probes, and lncRNA ISH/FISH probes. |
References
- Tachtatzis, P. M., et al. Chronic Hepatitis B Virus Infection: The Relation between Hepatitis B Antigen Expression, Telomere Length, Senescence, Inflammation and Fibrosis. PloS one. 2015. 10(5), e0127511.
- Chiang, A. J., et al. Detection of human papillomavirus in squamous cell carcinoma arising from dermoid cysts. Taiwanese journal of obstetrics & gynecology, 2015. 54(5), 559–566.
- Selimovic-Hamza, S., et al. Detection of Astrovirus in Historical Cases of European Sporadic Bovine Encephalitis, Switzerland 1958-1976. Frontiers in veterinary science, 2016. 3, 91.

