How Is the Cytotoxicity of Drugs Determined?
Through the twentieth century, the road from synthesizing a new drug molecule to becoming an actual product got longer than ever before. Cytotoxicity assays are a quick way to assess a certain chemical compound's effects on a given human cell line. Cytotoxicity experiments are a crucial part of the modern pharmaceutical development process. They are a cheap and safe way to get vital information about a new molecule's biological attributes focusing on its basic tolerability. These studies not only save human lives and test animals, but they also save the time and resources to be spared on a test molecule which is a complete failure having no in vitro safety.
Why Assess Cytotoxicity in Drug Development?
Cytotoxicity is a crucial consideration when developing new pharmaceutical products. Exposure to cytotoxic drugs can lead to a range of adverse effects, including tissue damage, organ dysfunction, and even life-threatening complications. Consequently, the evaluation of cytotoxicity is essential to ensure the safe use of potential drug candidates.
Furthermore, the assessment of cytotoxicity is not only a regulatory requirement for drug approval but also a crucial aspect of demonstrating the overall safety and tolerability of a new therapeutic. Regulatory agencies, such as the FDA and EMA, place a strong emphasis on the comprehensive evaluation of cytotoxicity as part of the drug approval process.
Introduction of Cell-Based Cytotoxicity Assays
The cellular damage caused by different chemical compounds can be various and thus, the methods to measure this effect are numerous. To select the proper test, we must know: the number of treated cells, the number of treatments, what kind of treatment the cells got, do we need these cells later, or if the chosen method can terminate them. The mechanism of cytotoxicity can be various so a single method only gives a simple view on a chosen material. Multiple tests and methods must be used before anyone can make a solid point about the biocompatibility of a chemical compound.
Assays
Cell viability assays are usually cheap, easy-to-perform methods, where after a given incubation with the selected chemical compound, the number of surviving cells is measured by some method. They use no antibodies or radioactive chemicals. Usually, these assays are carried out on 96- or 384-well plates, making them ideal for screening experiments.
| Name | Mechanism | How to detect |
| XTT | Enzymatic activity | Spectrophotometer |
| MTT | Enzymatic activity | Spectrophotometer |
| WST-1 | Enzymatic activity | Spectrophotometer |
| WST-8 | Enzymatic activity | Spectrophotometer |
| MTS | Enzymatic activity | Spectrophotometer |
| LDH | Enzymatic activity | Spectrophotometer |
| Resazurin | Enzymatic activity | Spectrophotometer |
| Neutral Red | Lysosomal uptake | Spectrophotometer |
RT-CES
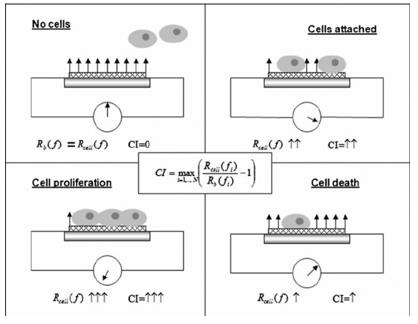 Fig.1 The mechanism of the RT-CES cell-survival assay. (Roa W, et al., 2011)
Fig.1 The mechanism of the RT-CES cell-survival assay. (Roa W, et al., 2011)
This technique is based on the impedance changes of the cell populations. The cells are seeded into special E-plates, which are available in multiple sizes (4-, 8-, 16-, 96-wells, etc.). These special devices have a positive and a negative electrode in every well, and a low voltage alternate current flows through the well. As the cells grow, they have a higher impedance (resistance in AC circuits); and as they die, the impedance value lowers. This effect has no impact on the cells, so it is a non-invasive technique. The length of the experiment is theoretically unlimited, as there is no end-point of AC flow. For this reason, cell growth can be measured during multiple treatments of the cells, and not just the cytotoxic or non-toxic effects, but the possible recovery of the cells can be studied as well. It is important, that first, a part of the cell medium and the solution of the screened chemical must be placed into the wells of the E-plate, thus the connected software can detect it as a background, with zero impedance, so chemicals with ionic charges do not interfere with the measured signal. The cells should be added to the wells after the background detection in a high-density suspension. Also, the whole experiment can be stopped at any point, to remove the test solution or to add a new compound to the test system.
What Factors Influence Cytotoxicity?
The cytotoxicity of a drug can be influenced by a variety of factors, including the concentration and exposure time of the compound, the specific cell type and its cell density, the metabolic activity and proliferation rate of the cells, and the underlying mechanism of action of the drug.
Drug concentration and exposure time
One of the primary factors affecting cytotoxicity is the concentration of the drug and the duration of exposure. In general, as the concentration of a cytotoxic compound increases, the likelihood of cell death or impaired cellular function also rises. Similarly, prolonged exposure to a drug can lead to accumulative cytotoxic effects, even at lower concentrations.
Cell type and cell density
The sensitivity of different cell types to a given drug can vary significantly, depending on factors such as the expression of specific receptors, the metabolic pathways, and the proliferation rate of the cells. For instance, rapidly dividing cells may be more susceptible to certain classes of drugs that target cell division or DNA replication.
Cellular metabolic activity and proliferation rate
The metabolic activity and proliferation rate of the target cells can also play a significant role in determining their susceptibility to cytotoxic insults. Cells with high metabolic activity or rapid proliferation may be more vulnerable to certain cytotoxic mechanisms, such as those that disrupt mitochondrial function or interfere with DNA replication.
Mechanism of action of the drug
The underlying mechanism of action of a drug can also contribute to its cytotoxic potential. Certain classes of compounds, such as those that target DNA synthesis, cell division, or intracellular signaling pathways, may inherently possess a higher risk of cytotoxicity compared to drugs with different mechanisms of action.
Creative Bioarray Relevant Recommendations
Reference
- Roa W, et al. (2011). "Real-time cell-impedance sensing assay as an alternative to clonogenic assay in evaluating cancer radiotherapy." Anal Bioanal Chem. 400 (7): 2003-11.

