- You are here: Home
- Disease Models
- Cardiovascular Disease Models
- Pulmonary Arterial Hypertension (PAH) Models
- Monocrotaline (MCT)-Induced Pulmonary Arterial Hypertension (PAH) Model
Disease Models
- Oncology Models
-
Inflammation & Autoimmune Disease Models
- Rheumatoid Arthritis Models
- Glomerulonephritis Models
- Multiple Sclerosis (MS) Models
- Ocular Inflammation Models
- Sjögren's Syndrome Model
- LPS-induced Acute Lung Injury Model
- Peritonitis Models
- Passive Cutaneous Anaphylaxis Model
- Delayed-Type Hypersensitivity (DTH) Models
- Inflammatory Bowel Disease Models
- Systemic Lupus Erythematosus Animal Models
- Asthma Model
- Sepsis Model
- Psoriasis Model
- Atopic Dermatitis (AD) Model
- Scleroderma Model
- Gouty Arthritis Model
- Carrageenan-Induced Air Pouch Synovitis Model
- Carrageenan-Induced Paw Edema Model
- Experimental Autoimmune Myasthenia Gravis (EAMG) Model
-
Cardiovascular Disease Models
- Surgical Models
- Animal Models of Hypertension
- Venous Thrombosis Model
- Atherosclerosis model
- Cardiac Arrhythmia Model
- Hyperlipoidemia Model
- Doxorubicin-induced Heart Failure Model
- Isoproterenol-induced Heart Failure Model
- Arterial Thrombosis Model
- Pulmonary Arterial Hypertension (PAH) Models
- Heart Failure with Preserved Ejection Fraction (HFpEF) Model
-
Neurological Disease Models
- Alzheimer's Disease Modeling and Assays
- Seizure Models
- Parkinson's Disease Models
- Ischemic Stroke Models
- Acute Spinal Cord Injury (ASCI) Model
- Traumatic Brain Injury (TBI) Model
- Hypoxic-Ischemic Encephalopathy (HIE) Model
- Tourette Syndrome (TS) Model
- Amyotrophic Lateral Sclerosis (ALS) Model
- Huntington's Disease (HD) Model
- Intracerebral hemorrhage (ICH) Models
- Pain Models
- Metabolic Disease Models
- Liver Disease Models
- Rare Disease Models
- Respiratory Disease Models
- Digestive Disease Models
-
Urology Disease Models
- Cisplatin-induced Nephrotoxicity Model
- Unilateral Ureteral Obstruction Model
- 5/6 Nephrectomy Model
- Renal Ischemia-Reperfusion Injury (RIRI) Model
- Diabetic Nephropathy (DN) Models
- Passive Heymann Nephritis (PHN) Model
- Adenine-Induced Chronic Kidney Disease (CKD) Model
- Kidney Stone Model
- Doxorubicin-Induced Nephropathy Model
- Orthopedic Disease Models
- Ocular Disease Models
- Skin Disease Models
- Infectious Disease Models
Monocrotaline (MCT)-Induced Pulmonary Arterial Hypertension (PAH) Model
Creative Bioarray has developed the monocrotaline (MCT)-induced pulmonary arterial hypertension (PAH) model that serves as an invaluable asset for our clients. This robust model enables our partners to screen potential drug candidates with exceptional efficiency, ensuring that the most promising compounds are identified for further research. Additionally, it provides a platform for discovering novel therapeutic targets in PAH, paving the way for innovative treatments and breakthroughs in this complex disease area. We invite researchers interested in PAH to learn more about our model and explore the opportunities it presents for advancing their research.
MCT is an 11‐membered macrocyclic pyrrolizidine alkaloid derived from the seeds of the Crotalaria spectabilis plant. In 1967, Crotalaria spectabilis was first reported to induce MCT-induced PAH in rats. This was accompanied by right ventricular hypertrophy and an increase in the thickness of the pulmonary arteries' medial layer. Since then, the MCT-induced PAH model has gained popularity due to its simplicity, reproducibility, and affordability. MCT is metabolized by liver cytochrome P450 3A4 (CYP3A4) into the toxic metabolite MCT pyrrole (MCTP). This, in turn, damages vascular endothelial cells (ECs) and leads to inflammation.
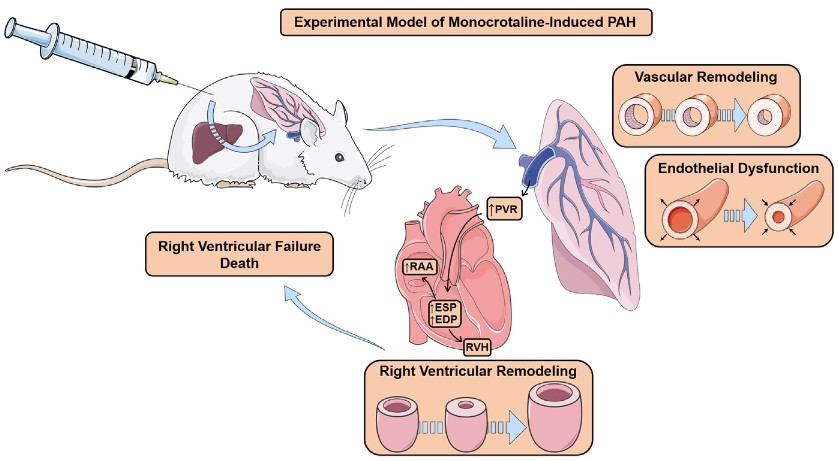 Fig. 1 Experimental model of monocrotaline-induced pulmonary arterial hypertension (PAH). After monocrotaline injection, rats undergo vascular remodelling and endothelial dysfunction, which lead to a progressive increase in pulmonary vascular resistance (PVR) and, ultimately, to right ventricular hypertrophy (RVH) and increased RV systolic and diastolic pressures, as well as an increase in right atrial area (RAA). Due to the progressive increase in PVR, RV function deteriorates over time and, eventually, the animal dies of RV failure. ESP: end-systolic pressure; EDP: end-diastolic pressure. (Santos-Ribeiro, D et al. 2016)
Fig. 1 Experimental model of monocrotaline-induced pulmonary arterial hypertension (PAH). After monocrotaline injection, rats undergo vascular remodelling and endothelial dysfunction, which lead to a progressive increase in pulmonary vascular resistance (PVR) and, ultimately, to right ventricular hypertrophy (RVH) and increased RV systolic and diastolic pressures, as well as an increase in right atrial area (RAA). Due to the progressive increase in PVR, RV function deteriorates over time and, eventually, the animal dies of RV failure. ESP: end-systolic pressure; EDP: end-diastolic pressure. (Santos-Ribeiro, D et al. 2016)
Our Monocrotaline (MCT)-Induced Pulmonary Arterial Hypertension (PAH) Model
- Available Animal
- Rat
- Modeling Method
Adult male rats receive a single subcutaneous/intraperitoneal injection of MCT to induce PAH model.
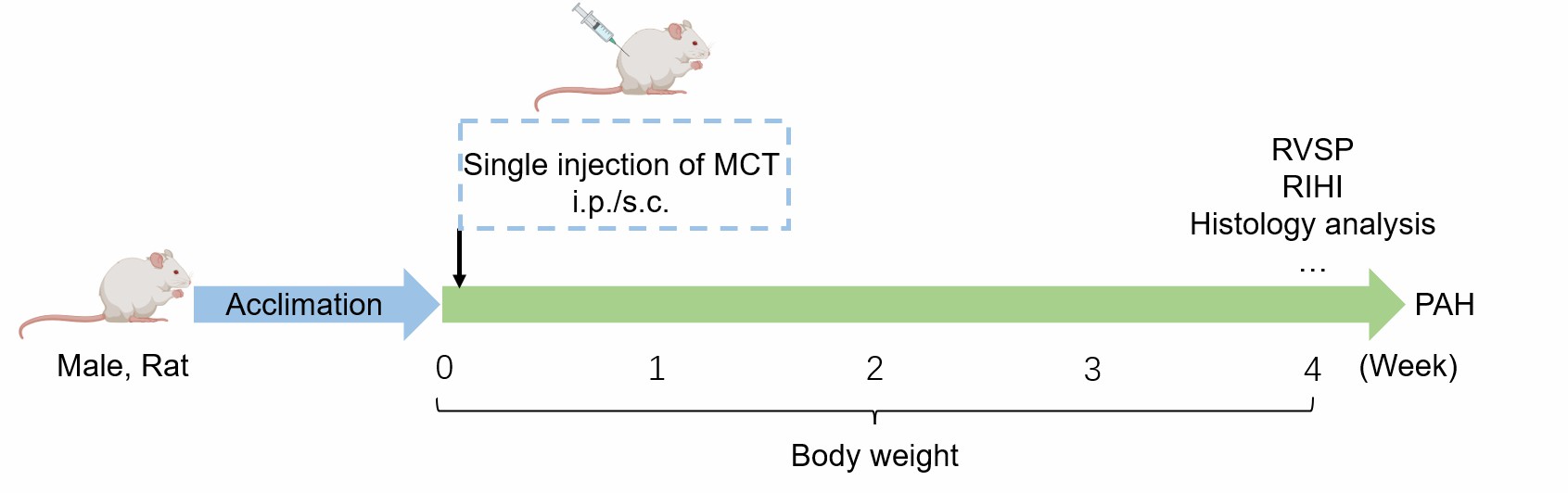 Fig. 2 Schematic diagram of MCT-induced PAH model.
Fig. 2 Schematic diagram of MCT-induced PAH model.
- Endpoints
- Right ventricular systolic pressure (RVSP)
- Right ventricular hypertrophy index (RVHI)
- Body weight
- Tissue weight: heart, lung
- Histology analysis (lung tissue): H&E staining, Sirius red staining, Masson trichrome staining, etc
- qPCR or Western blot
- Other customized endpoints
Example Data
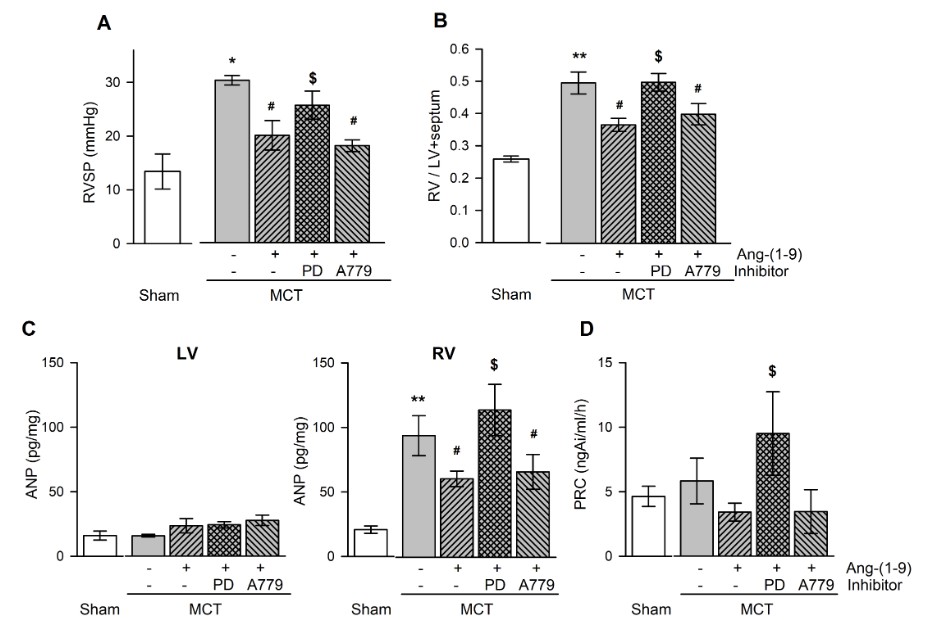 Fig. 3 Effects of Ang-(1-9) on ventricular pressure and weight, and plasma hormone levels in MCT-treated rats. Changes in RVSP (A), RV/LV+septum (B), ANP in ventricular tissue (C), and PRC (D) with and without Ang-(1-9) in MCT-treated rats. RVSP, right ventricular systolic pressure; RV/LV+septum, the ratio of free wall of right ventricular weight to left ventricular weight+septal weight; PRC, plasma renin concentration; MCT, monocrotaline; LV, RV, left and right ventricle, respectively; PD, PD123,319. (Cha et al. 2018)
Fig. 3 Effects of Ang-(1-9) on ventricular pressure and weight, and plasma hormone levels in MCT-treated rats. Changes in RVSP (A), RV/LV+septum (B), ANP in ventricular tissue (C), and PRC (D) with and without Ang-(1-9) in MCT-treated rats. RVSP, right ventricular systolic pressure; RV/LV+septum, the ratio of free wall of right ventricular weight to left ventricular weight+septal weight; PRC, plasma renin concentration; MCT, monocrotaline; LV, RV, left and right ventricle, respectively; PD, PD123,319. (Cha et al. 2018)
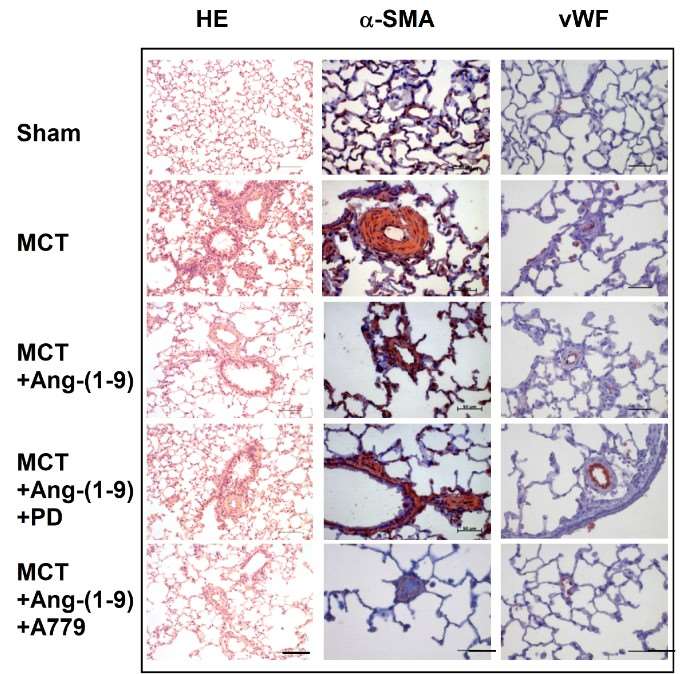 Fig. 4 Effects of Ang-(1-9) on MCT-induced pulmonary arterial intimal proliferation. (Cha et al. 2018)
Fig. 4 Effects of Ang-(1-9) on MCT-induced pulmonary arterial intimal proliferation. (Cha et al. 2018)
In addition, we also provide another PAH model that maybe you are interested in:
Quotation and Ordering
Creative Bioarray is delighted to offer our state-of-the-art technology and extensive experience in rodent disease models to support our clients' research endeavors and project advancement. If you are interested in our services, please feel free to contact us at any time or submit an inquiry to us directly.
References
- Santos-Ribeiro, D., et al. Pulmonary arterial hypertension: Basic knowledge for clinicians. Archives of cardiovascular diseases, 2016, 109(10): 550-561.
- Cha, S.A., et al. Angiotensin-(1-9) ameliorates pulmonary arterial hypertension via angiotensin type II receptor. The Korean journal of physiology & pharmacology: official journal of the Korean Physiological Society and the Korean Society of Pharmacology, 2018, 22(4): 447.
- Wu, X.H., et al. Experimental animal models of pulmonary hypertension: Development and challenges. Animal Model Exp Med, 2022;5(3):207-216.
For research use only. Not for any other purpose.

