Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
SW-900
Cat.No.: CSC-C9732L
Species: Human
Source: lung
Morphology: epithelial
Culture Properties: monolayer
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Tumorigenecity: Yes, produces tumors in nude mice consistent with type II bronchiolar adenocarcinoma
Isoenzyme: G6PD, B;PGM1,1;PGM3,2;ES-D,1;Me-2,2;AK-1,1;GLO-1,2
Karyology: hypotriploid
Histopathology: carcinoma, squamous cell; grade IV
vWA: 16
FGA: 21,23
Amelogenin: X
TH01: 8
TPOX: 11
CSF1P0: 11
D5S818: 11
D13S317: 8
D7S820: 11,12
Shipping Condition: Room Temperature
The SW-900 cell line is a human lung cancer cell line established from the tumor of a 53-year-old Caucasian male diagnosed with type II bronchiolar adenocarcinoma. This specific origin provides a critical resource for researchers studying the biological and molecular aspects of lung adenocarcinomas, particularly those that exhibit aggressive characteristics. The SW-900 cell line is significant for its tumorigenic properties, as it has been shown to form tumors in nude mice that mirror the original tumor's morphology and histopathology. This ability to replicate the disease in a live model makes SW-900 a valuable tool for in vivo studies of lung cancer progression and therapeutic responses.
Histologically, SW-900 is classified as a grade IV squamous cell carcinoma, indicating a poorly differentiated tumor with high malignancy. This classification is essential for understanding the aggressive nature of the cancer and its potential treatment challenges. The poorly differentiated status suggests that the cells have lost many characteristics of normal squamous cells, leading to increased invasiveness and resistance to standard therapies. Researchers utilize this cell line to investigate the mechanisms driving tumor aggressiveness and to explore novel therapeutic approaches that may improve outcomes for patients with advanced lung cancer. Karyotypically, SW-900 is classified as hypotriploid, indicating an abnormal chromosomal composition, which is a common feature in cancer cells. This chromosomal instability contributes to the genetic diversity within tumors, potentially affecting their behavior and responsiveness to therapies.
Induction of Migration, Invasion, and EMT of SW-900 Cells
Serine/threonine/tyrosine kinase 1 (STYK1) has been previously shown to have oncogenic properties, and emerging evidence suggests that STYK1 expression correlates with epithelial-mesenchymal transition (EMT). To investigate whether STYK1 induced migration, invasion, and EMT, lentiviruses were used to augment STYK1 expression in SW-900 cells. Significant overexpression of STYK1 was observed by RT-qPCR and immunoblotting in SW-900 cells after their transduction with lentiviruses carrying STYK1 (Fig. 1A-C). After lentiviral transduction, enhanced cell migration and invasion capacities were also observed using the RTCA assay and crystal violet staining (Fig. 1D-I). In addition, the results of immunoblots and semiquantitative analysis showed that overexpression of STYK1 in SW-900 cells promoted EMT, as was evidenced by increases in the expression of Snail, ZEB2, and vimentin, and a decrease in E-cadherin expression (Fig. 1J-N).
To assess the effect of STYK1 on the metastasis of NSCLC cells in vivo, wild-type and STYK1 overexpressing SW-900 cells were transfected with plasmids carrying luciferase and injected via the tail vein into nude mice to construct a xenograft metastatic NSCLC model. The results of in vivo imaging revealed that 4 weeks after tail vein injection of tumor cells, mice harboring STYK1 overexpressing SW-900 cells had more metastatic sites (Fig. 2A) and emitted more photons than mice injected with wild-type cells (Fig. 2B). Such differences were much more significant on week 5 after tumor cell injection (Fig. 2C, D). Furthermore, hematoxylin/eosin staining showed that lung metastatic foci in mice injected with STYK1 overexpressing SW-900 cells outnumbered those in mice that received wild-type SW-900 cells (Fig. 2E, F). These results demonstrate that the upregulation of STYK1 expression in NSCLC cells promoted metastasis in vivo.
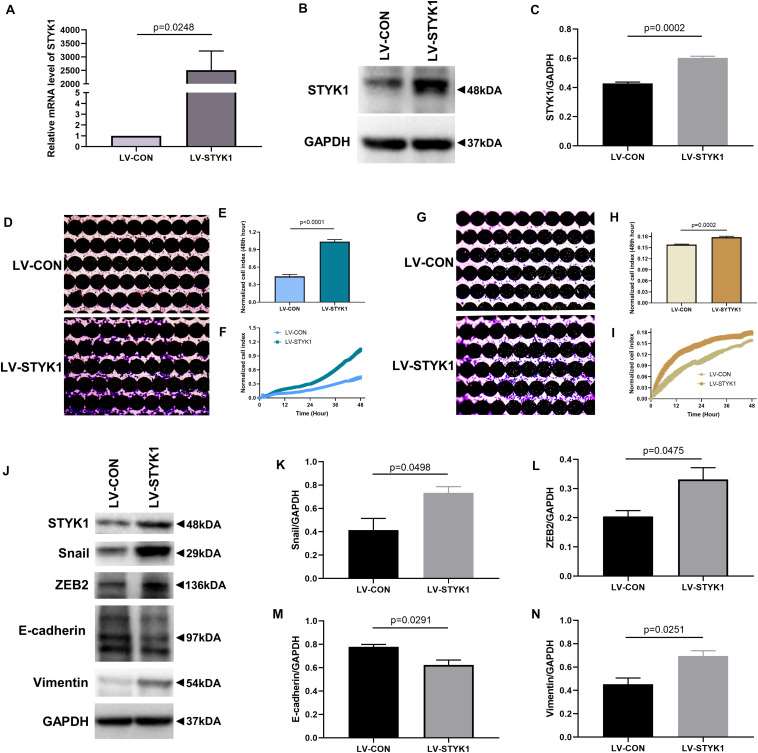 Fig. 1 STYK1 overexpression upregulates migration, invasion, and EMT in SW-900 cells. (A-C) STYK1 expression in SW-900 cells after transduction with STYK1 encoding lentiviruses. (D) Capacity for cell migration after transduction with lentiviruses encoding STYK1. (E) Normalized cell index after lentiviral STYK1 overexpression at the endpoint of RTCA. (F) Cell index curves after lentiviral STYK1 overexpression. (G-I) Cell invasion capacity after lentiviral STYK1 overexpression evaluated by RTCA and crystal violet staining. (J–N) Typical immunoblots and semiquantitative analysis of EMT biomarkers after transduction with lentiviruses encoding STYK1. (Lai Y, et al., 2021)
Fig. 1 STYK1 overexpression upregulates migration, invasion, and EMT in SW-900 cells. (A-C) STYK1 expression in SW-900 cells after transduction with STYK1 encoding lentiviruses. (D) Capacity for cell migration after transduction with lentiviruses encoding STYK1. (E) Normalized cell index after lentiviral STYK1 overexpression at the endpoint of RTCA. (F) Cell index curves after lentiviral STYK1 overexpression. (G-I) Cell invasion capacity after lentiviral STYK1 overexpression evaluated by RTCA and crystal violet staining. (J–N) Typical immunoblots and semiquantitative analysis of EMT biomarkers after transduction with lentiviruses encoding STYK1. (Lai Y, et al., 2021)
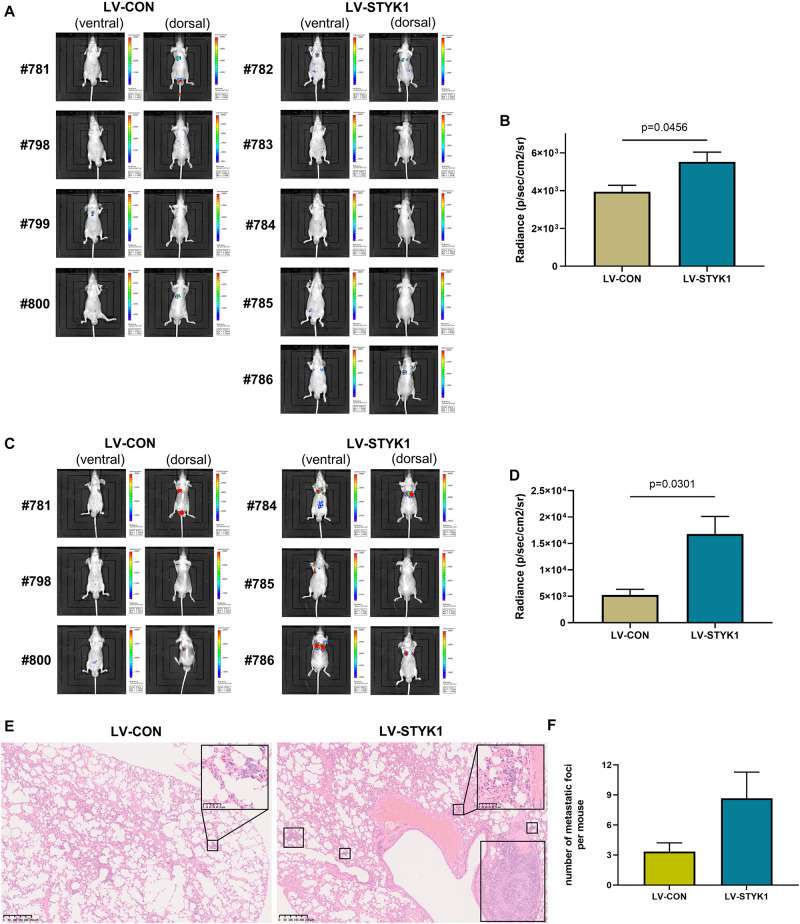 Fig. 2 Overexpression of STYK1 promotes metastasis of SW-900 cells in nude mice. (A) Luminescent images of nude mice in 4 weeks after tail vein injection of SW-900 cells. (B) Quantitative analysis of radiance in 4 weeks after tail vein injection of SW-900 cells. (C, D) Luminescent images of nude mice and quantification of radiance in 5 weeks after tail vein injection of SW-900 cells. (E) Representative histological sections of lung metastatic lesions. (F) Comparison of the number of lung metastasis between groups. (Lai Y, et al., 2021)
Fig. 2 Overexpression of STYK1 promotes metastasis of SW-900 cells in nude mice. (A) Luminescent images of nude mice in 4 weeks after tail vein injection of SW-900 cells. (B) Quantitative analysis of radiance in 4 weeks after tail vein injection of SW-900 cells. (C, D) Luminescent images of nude mice and quantification of radiance in 5 weeks after tail vein injection of SW-900 cells. (E) Representative histological sections of lung metastatic lesions. (F) Comparison of the number of lung metastasis between groups. (Lai Y, et al., 2021)
si-HIF-1α Inhibits Malignant Progression in NSCLC Cells
Non-small cell lung cancer (NSCLC) is the most common type of cancer, accounting for ~85% of all cases of lung cancer, and is a major cause of cancer-associated mortality worldwide. Ferroptosis and hypoxia-inducible factor 1α (HIF-1α) have critical roles in human tumors. The expression levels of HIF-1α in SW-900 and A549 NSCLC cell lines and BEAS-2B normal bronchial epithelial cells were detected using RT-qPCR and western blotting. The mRNA and protein expression levels of HIF-1α in the NSCLC cells were significantly higher than those in the normal cells (P<0.01). In addition, the mRNA and protein expression levels of HIF-1α in A549 cells were higher than those in SW-900 cells (P<0.05; Fig. 3A and B). The knockdown of HIF-1α in NSCLC cells by transfection with si-HIF-1α significantly decreased the mRNA and protein expression levels of HIF-1α compared with the respective levels in cells transfected with si-NC (P<0.01; Fig. 3C and D).
The effects of HIF-1α silencing on the malignant progression of NSCLC were investigated. The CCK-8 assay showed that HIF-1α silencing significantly suppressed the proliferation of SW-900 and A549 cells compared with the proliferation in the respective si-NC group (P<0.05; Fig. 4A). Additionally, the flow cytometry results showed that the silencing of HIF-1α significantly increased the apoptosis of SW-900 and A549 cells (P<0.01; Fig. 4B). Moreover, the knockdown of HIF-1α significantly decreased the invasion ability of SW-900 and A549 cells compared with that of the SW-900 and A549 cells transfected with si-NC (P<0.01; Fig. 4C).
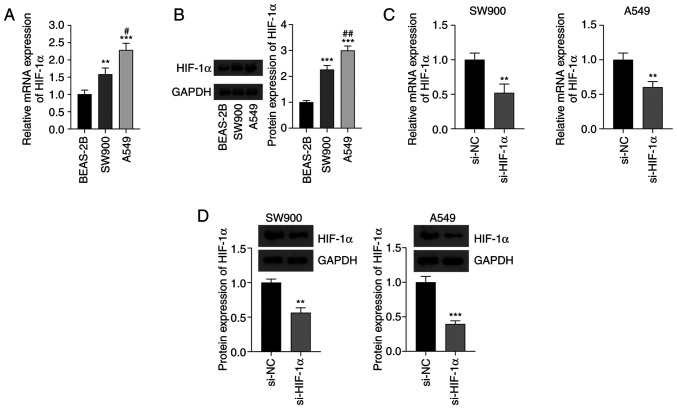 Fig. 3 Expression of HIF-1α in NSCLC cells. (A) Expression of HIF-1α mRNA in SW-900 and A549 NSCLC cell lines and normal BEAS-2B cells determined using reverse transcription-quantitative polymerase chain reaction. (B) Results of the western blot analysis of HIF-1α protein. (C) mRNA and (D) protein expression of HIF-1α in NSCLC cells transfected with si-HIF-1α. (Zheng S, et al., 2023)
Fig. 3 Expression of HIF-1α in NSCLC cells. (A) Expression of HIF-1α mRNA in SW-900 and A549 NSCLC cell lines and normal BEAS-2B cells determined using reverse transcription-quantitative polymerase chain reaction. (B) Results of the western blot analysis of HIF-1α protein. (C) mRNA and (D) protein expression of HIF-1α in NSCLC cells transfected with si-HIF-1α. (Zheng S, et al., 2023)
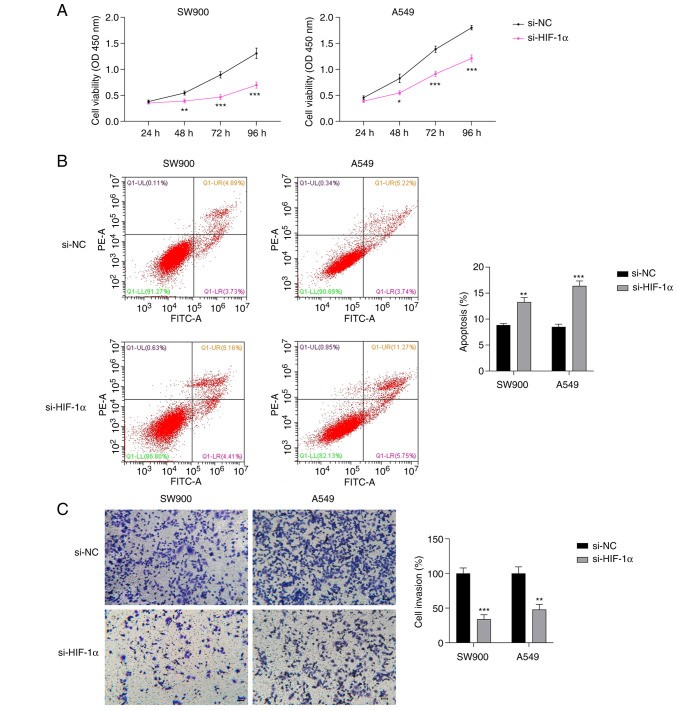 Fig. 4 Effect of si-HIF-1α on the proliferation, invasion, and ferroptosis of NSCLC cells. Results of (A) Cell Counting Kit-8, (B) flow cytometry, and (C) Transwell invasion assays of NSCLC cells transfected with si-HIF-1α. (Zheng S, et al., 2023)
Fig. 4 Effect of si-HIF-1α on the proliferation, invasion, and ferroptosis of NSCLC cells. Results of (A) Cell Counting Kit-8, (B) flow cytometry, and (C) Transwell invasion assays of NSCLC cells transfected with si-HIF-1α. (Zheng S, et al., 2023)
The matrigel plug angiogenesis assay is a simple in vivo technique to detect the newly formed blood vessels in the transplanted gel plugs in nude mice. The matrigel matrix is derived from the engelbroth-holm-swarm (EHS) mouse sarcoma, and its composition is comparable to the basement membrane proteins.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
Invaluable
These cells were invaluable in studying the development of resistance and may contribute to the design of more effective therapies.
19 Feb 2023
Ease of use
After sales services
Value for money
Write your own review
- You May Also Need