Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
MES-SA/Dx-5
Cat.No.: CSC-C9508J
Species: Human
Source: Reproductive: Uterus
Morphology: Epithelial
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can cryopreserved cells be kept at -20 °C.
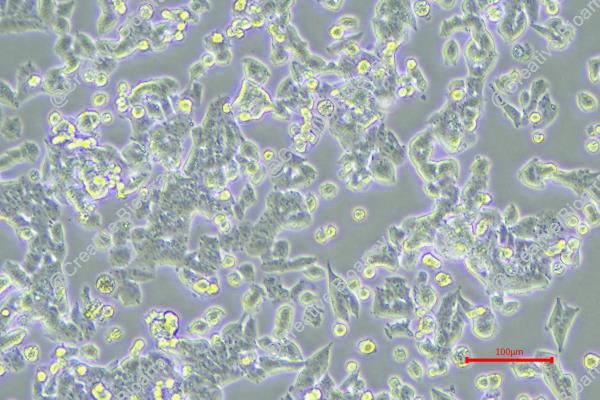
The MES-SA/Dx-5 cell line is derived from tumor tissue of a 56-year-old Caucasian female during a hysterectomy. It is a variant of the MES-SA cell line, originally obtained from MES-SA cells through drug screening with doxorubicin. MES-SA/Dx-5 cells are 100 times more resistant to doxorubicin (P-glycoprotein resistance). The P-glycoprotein is an ATP-dependent efflux pump that can transfer chemotherapeutic agents from the cell to the extracellular environment and confers drug resistance. MES-SA/Dx-5 cells also display extensive cross-resistance to many chemotherapeutic agents, including daunorubicin, docetaxel, vincristine, paclitaxel and colchicine, as well as moderate cross-resistance to mitomycin C and melphalan.
Morphologically, the MES-SA/Dx-5 line is epithelial or fibroblast-like, and adherent in vitro, with a doubling time of approximately 30 hours. Given its resistance to many chemotherapeutic drugs, scientists often experiment with a huge variety of compounds, from non-steroidal anti-inflammatory medications to quinoline derivatives, to determine if they can overcome P-glycoprotein-mediated resistance. These studies also address the antitumor activity and toxicities of novel compounds, and provide experimental information and strategies for designing novel antitumor drugs.
Antiproliferative Activity of Novel Double-Modified Derivatives of the Polyether Ionophore Monensin A
Monensin A (MON) is a naturally occurring ionophore antibiotic with extensive biological activity, including antibacterial, antifungal, and antiviral effects. MON's ability to disturb ion gradients in cells has shown potential for antiproliferative activity against various cancers. Its modification could lead to derivatives with enhanced biological activity and reduced toxicity. Klejborowska's team employed a novel one-pot synthesis method using triphosgene to create MON derivatives.
These derivatives were tested for their antiproliferative activity against two drug-sensitive (MES-SA, LoVo) and two drug-resistant (MES-SA/DX5, LoVo/DX) cancer cell lines. The mean IC50 ± SD of the compounds is in figure 1. MON had low IC50 values for MES-SA and MES-SA/DX5 (0.34 and 0.16 μM). MON derivatives were less effective against MES-SA than doxorubicin, cisplatin, and MON. However, their effectiveness increased significantly for MES-SA/DX5. Similar results were found for the LoVo cell line and its resistant subline (LoVo/DX). Only compound 1 was more effective against LoVo (IC50 = 0.66 μM) than doxorubicin (IC50 = 1.03 μM) and cisplatin (IC50 = 4.37 μM). MON derivatives were less toxic to normal fibroblasts (BALB/3T3) compared to the two reference compounds (IC50 = 8.06–76.23 μM for MON derivatives, 6.77 μM for cisplatin, and 0.72 μM for doxorubicin). Five derivatives (4–7,14) were inactive against BALB/3T3 cells at tested concentrations.
Resistance index (RI) values showed that many MON derivatives overcame doxorubicin resistance (Fig. 2). Twelve derivatives were equally or more effective on MES-SA/DX5 than MES-SA with RI values lower than reference materials (RI = 0.11–0.46). Thirteen derivatives were similarly effective on LoVo/DX compared to LoVo with lower RI values (RI = 0.28–0.97). The selectivity indices (SI) for therapeutic potential are in Table 2, showing most compounds (except 11) targeted cancer cells, particularly drug-resistant ones, over normal cells (SI = 1.07–2.60 for MES-SA, 2.72–17.59 for MES-SA/DX5, 0.85–12.21 for LoVo, 1.33–12.21 for LoVo/DX).
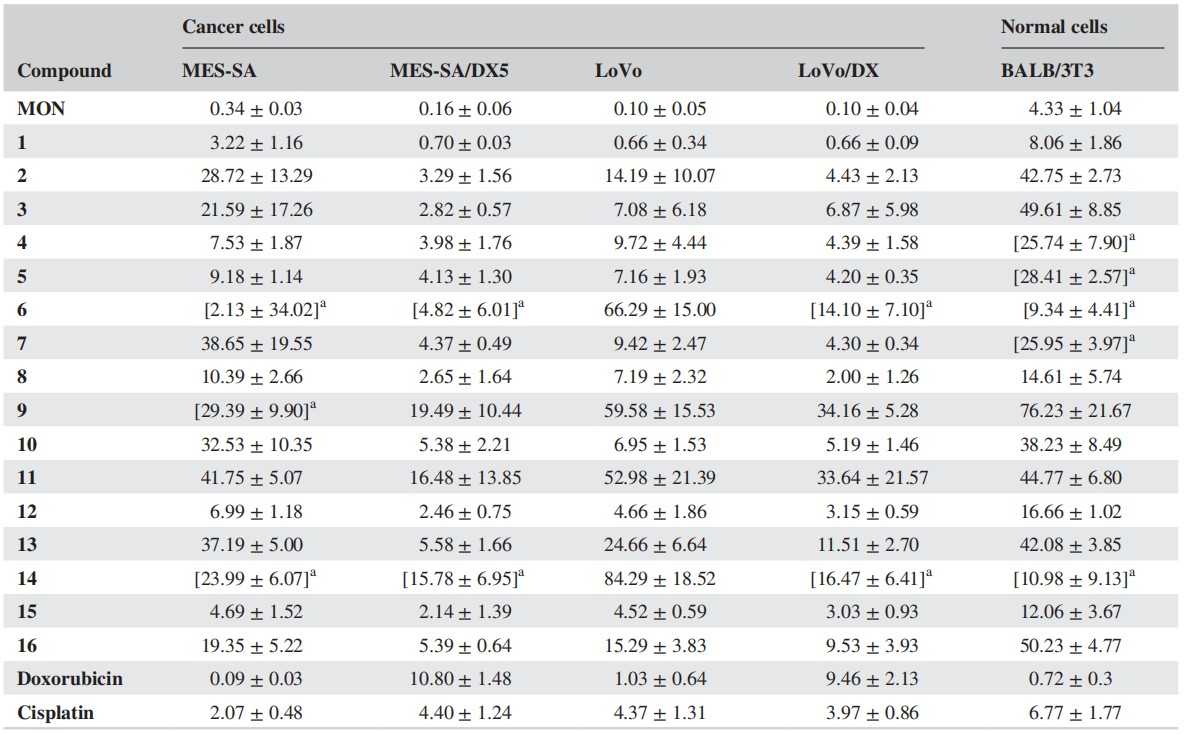 Fig. 1. Antiproliferative activity of MON and its derivatives (1–16) (Klejborowska G, Maj E, et al., 2018).
Fig. 1. Antiproliferative activity of MON and its derivatives (1–16) (Klejborowska G, Maj E, et al., 2018).
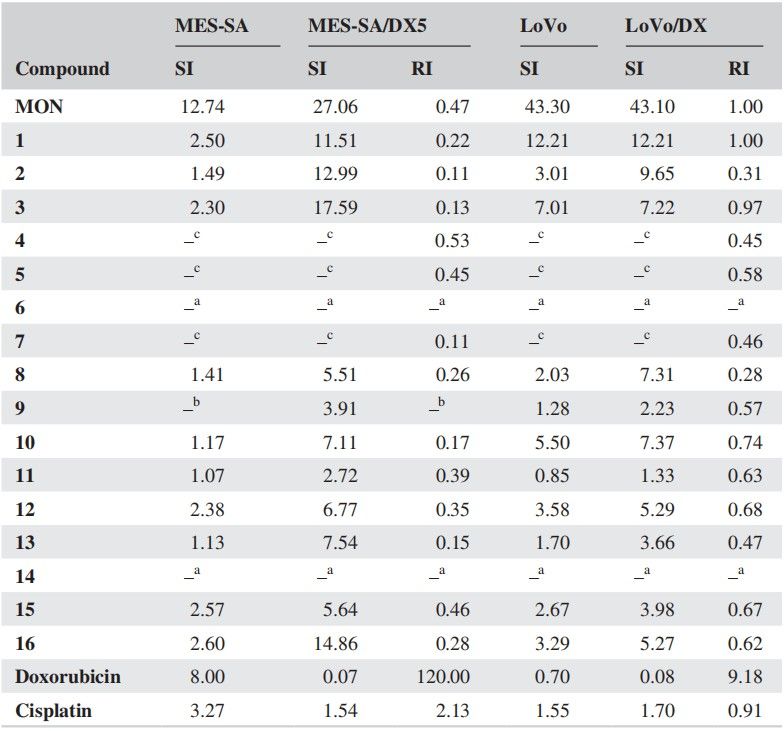 Fig. 2. The calculated values of the resistance index (RI) and selectivity (SI) of compounds tested (Klejborowska G, Maj E, et al., 2018).
Fig. 2. The calculated values of the resistance index (RI) and selectivity (SI) of compounds tested (Klejborowska G, Maj E, et al., 2018).
In Vitro Cytotoxicity of Nanomicelles in MCF7, 4T1 and Multidrug Resistant Uterine Cancer Cell Lines (MCF-7, MES-SA/DX5)
Cancer is the second leading cause of death in the world, and chemotherapy is the most common treatment. Low water solubility and resistance of tumors pose problems for paclitaxel, a powerful anti-tumour drug. To circumvent these barriers, Mostoufi's team made a series of di-block and tri-block pH-sensitive hybrid copolymers, PEG-PBLG, PEG-PLeu, PGA-PLeu and PEG-PGA-PLeu in varying compositions to deliver PTX.
The cytotoxicity of these nanomicelles was investigated in MCF7, 4T1 and multidrug resistant uterine cancer cell lines (MCF-7, MES-SA/DX5, respectively). These cytotoxicity tests revealed that free PTX and PTX-NPs inhibited the growth of MCF-7, 4T1 and MES-SA/DX5 cells at different concentrations and over different periods of time. PEG-PGA-PLeu 113-100-45 PTX-NPs performed better on MCF7 and 4T1 cells, IC50 of 7 and 60 ng/mL, compared with free PTX and other preparations (Fig. 3 A and B). For drug-resistant MES-SA/DX-5 cells, PEG-PGA-PLeu 113-100-45 PTX-NPs exhibited a significantly lower IC50 of 29 ng/mL, surpassing the others with a 7 to 12-fold increase in toxicity (Fig. 3C). This is partly due to different cell entry mechanisms: PTX-NPs are internalized via endocytosis, bypassing efflux transporters. The enhanced toxicity of nanoparticles is also attributed to LMP induction and cell apoptosis, related to PLeu in the copolymer. Additionally, PEG-PGA-PLeu 113-100-45 PTX-NPs were more pH-responsive than other copolymers, releasing more drug at acidic pH. Notably, bare copolymers showed no cytotoxicity (Fig. 4). The time-dependency of toxicity was evident with increased efficacy over time (Fig. 5). In conclusion, PEG-PGA-PLeu 113-100-45 PTX nanoparticles showed higher toxicity against drug-resistant cells compared to free PTX.
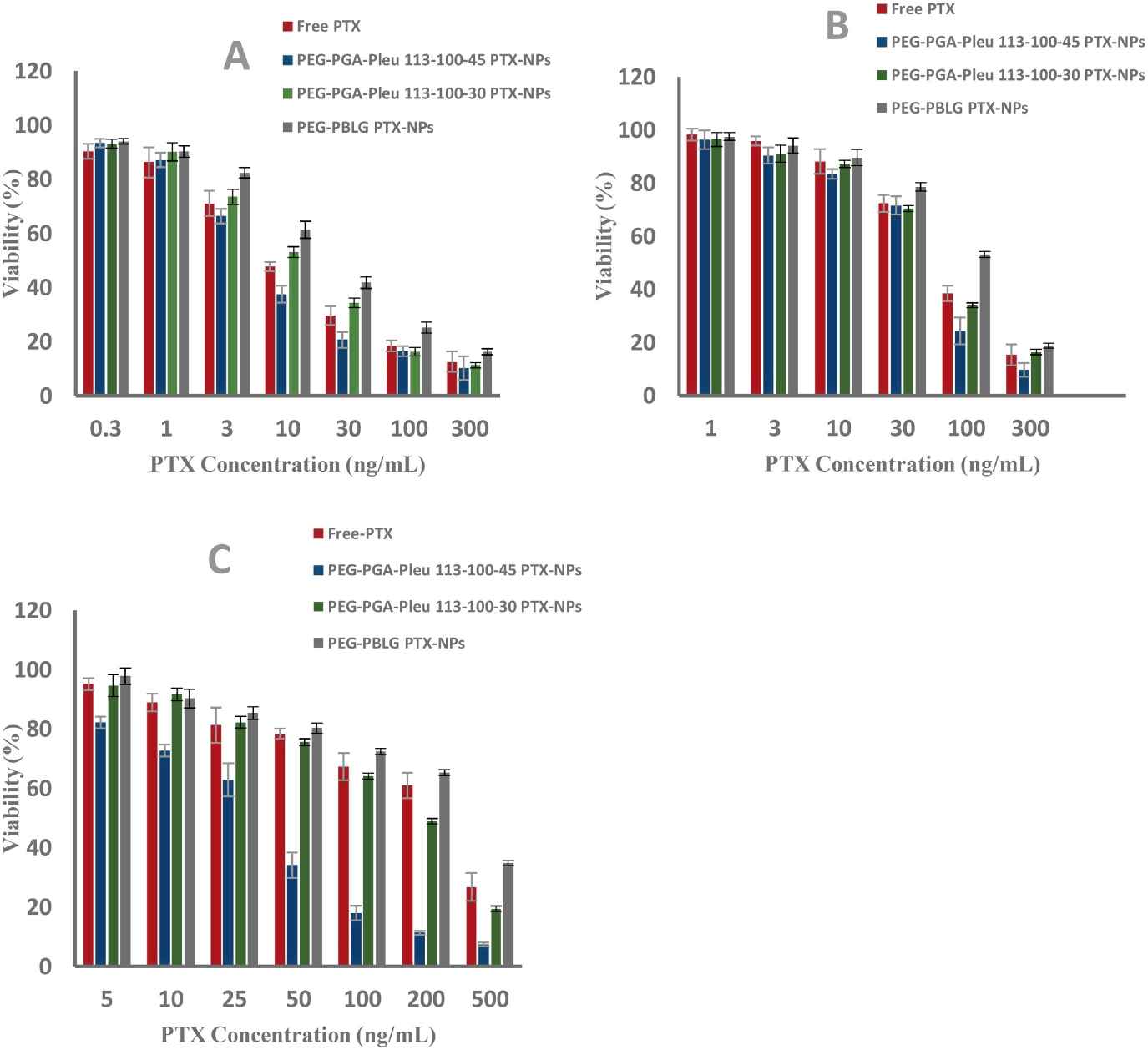 Fig. 3. Antitumor activity of free PTX, PTX-NPs 113-100-45, 113-100-30 and PEG-PBLG loaded PTX-NPs on (A)-MCF7 cells, (B)-4T1 cells, (C)-MES-SA/DX5 cells, determined by MTT assay (Mostoufi H, Yousefi G, et al., 2019).
Fig. 3. Antitumor activity of free PTX, PTX-NPs 113-100-45, 113-100-30 and PEG-PBLG loaded PTX-NPs on (A)-MCF7 cells, (B)-4T1 cells, (C)-MES-SA/DX5 cells, determined by MTT assay (Mostoufi H, Yousefi G, et al., 2019).
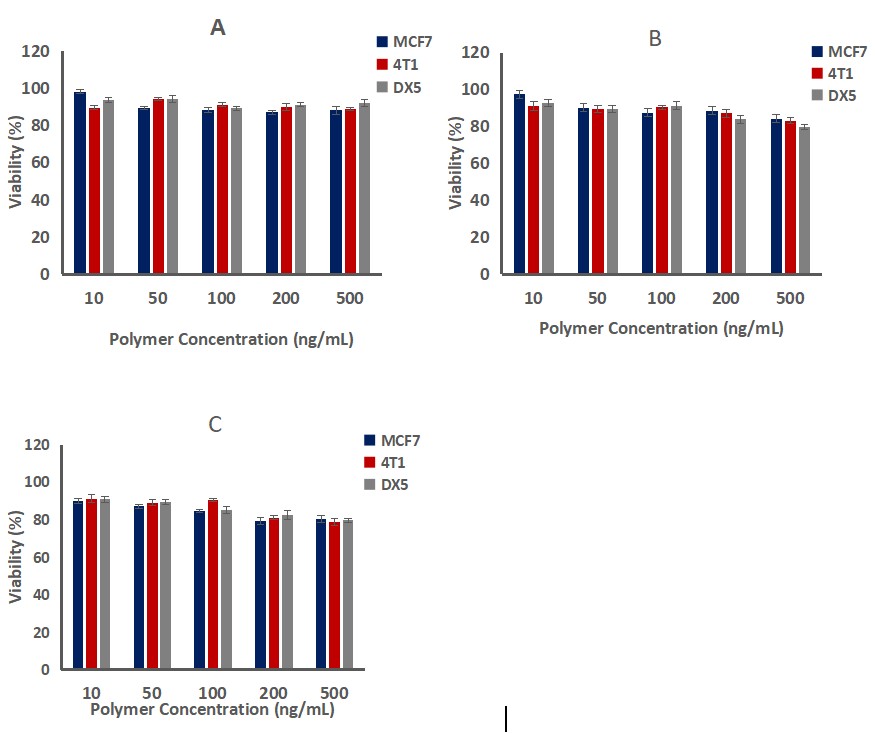 Fig. 4. The results of bare copolymers PEG-PGA-PLeu 113-100-45 (A), 113-100-30 (B) and PEG-PBLG (C) cytotoxicity against MCF7, 4T1 and MES-SA/DX5 (Mostoufi H, Yousefi G, et al., 2019).
Fig. 4. The results of bare copolymers PEG-PGA-PLeu 113-100-45 (A), 113-100-30 (B) and PEG-PBLG (C) cytotoxicity against MCF7, 4T1 and MES-SA/DX5 (Mostoufi H, Yousefi G, et al., 2019).
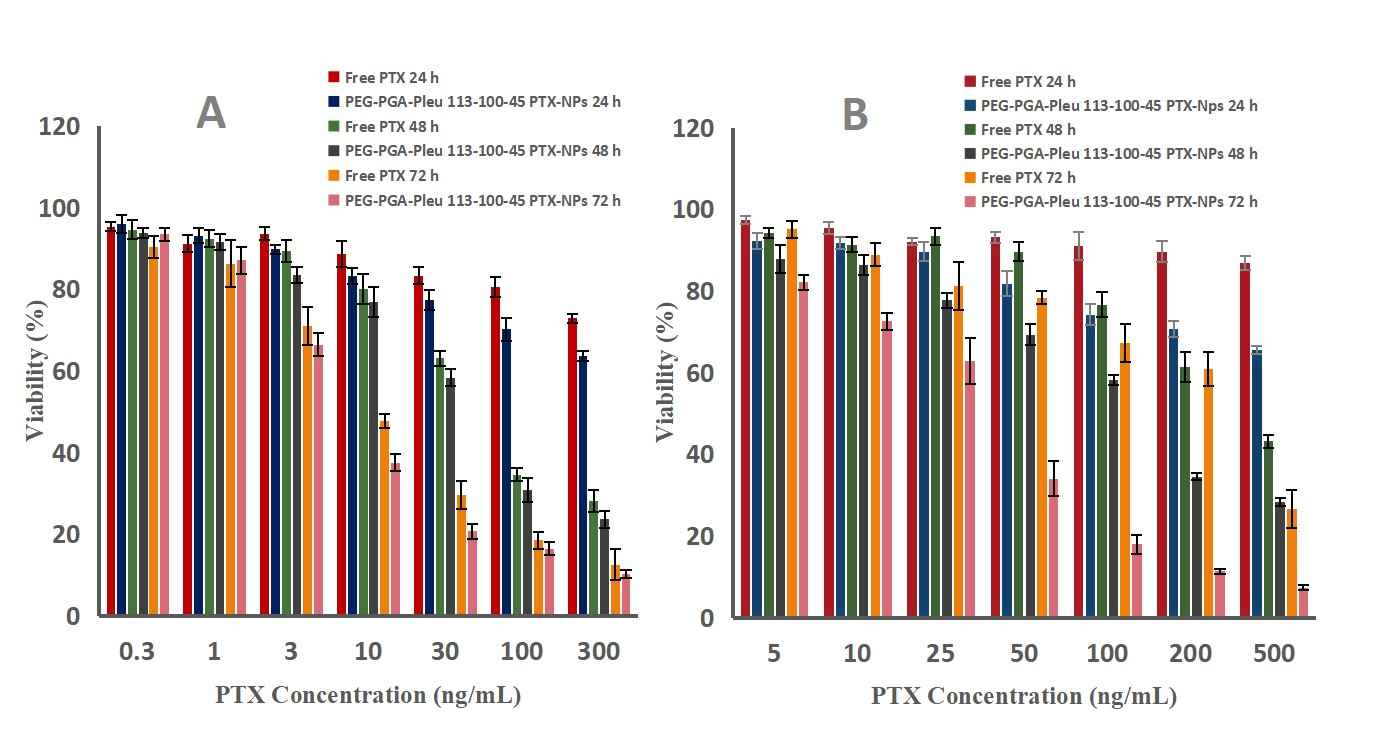 Fig. 5. Antitumor activity of free PTX and PTX-NPs 113-100-45 on (A)-MCF-7 cells, (B)- MES-SA/DX5 cells, determined by MTT assay (Mostoufi H, Yousefi G, et al., 2019).
Fig. 5. Antitumor activity of free PTX and PTX-NPs 113-100-45 on (A)-MCF-7 cells, (B)- MES-SA/DX5 cells, determined by MTT assay (Mostoufi H, Yousefi G, et al., 2019).
Challenges include the complex nature of tumor metastasis, variability in individual patient responses, and the need to identify reliable predictive markers.
Ask a Question
Average Rating: 4.0 | 1 Scientist has reviewed this product
Exceptional quality
The cell biology products greatly enhanced our research, delivering high-quality and consistent results across various experiments.
09 Feb 2023
Ease of use
After sales services
Value for money
Write your own review