Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
LCLC-103H
Cat.No.: CSC-C0432
Species: Human
Source: large cell lung carcinoma
Morphology: pleomorphic, gigantic, adherent cells growing as monolayer
Culture Properties: monolayer
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Immunology: cytokeratin +, cytokeratin-7 -, cytokeratin-8 +, cytokeratin-17 -, cytokeratin-18 +, cytokeratin-19 +, desmin -, endothel -, EpCAM -, GFAP -, neurofilament -
The LCLC-103H cell line is derived from the pleural effusion of a 61-year-old Caucasian male patient diagnosed with large cell lung carcinoma (LCLC) featuring giant cells. This cell line was established to provide a model for studying the unique characteristics of large-cell lung carcinoma, a subtype of non-small cell lung cancer that is known for its aggressive behavior and poor prognosis. The establishment of LCLC-103H allows researchers to explore the biology of this cancer type, particularly in the context of pleural effusions, which are common complications in advanced lung cancer cases.
One of the notable features of LCLC-103H is its remarkable ability to form stroma, which is the supportive tissue surrounding tumor cells. This stroma formation is significant as it plays a critical role in tumor progression, influencing factors such as nutrient supply, immune evasion, and metastasis. The presence of giant cells in this cell line also provides an opportunity to investigate the specific mechanisms associated with this variant of large-cell carcinoma, which may exhibit different biological behaviors compared to more typical large-cell tumors.
Additionally, LCLC-103H is characterized by the overexpression of the proto-oncogene MYC. This overexpression is particularly relevant as MYC is known to be involved in cell proliferation, differentiation, and apoptosis. The dysregulation of MYC signaling is commonly associated with various cancers, including lung carcinoma, and contributes to tumor aggressiveness and treatment resistance. Researchers utilize LCLC-103H to study the implications of MYC overexpression in large-cell lung carcinoma, including its impact on therapeutic responses and tumor microenvironment interactions.
Canonical BMP Signaling Pathway Is Active in LCLC‑103H Lung Cancer Cells
Bone morphogenetic protein (BMP) signaling has an oncogenic and tumor-suppressing activity in lung cancer that makes the prospective therapeutic utility of BMP signaling in lung cancer treatment complex. BMP-4 treatment of LCLC-103H lung cancer cells triggered the activation of canonical SMAD-dependent BMP signaling. This was confirmed by the detection of a strong increase in the phosphorylated form of SMAD1, 5, and 9 (pSMAD1/5/9) as early as 30 min after the treatment (Fig. 1a, b). Using Western blot analysis, the phosphorylation events were detected with 50 ng/ml and 100 ng/ml, but not with 2 ng/ml BMP-4 concentration. The increase in the amount of pSMAD1/5/9 stayed significant even 24 hours after the treatment, compared to the vehicle control cells. The activation of SMAD-dependent signaling was successfully blocked by the BMP receptor type I antagonist, LDN-214117 when used alone or as a pre-treatment before BMP-4 stimulation.
The ability of BMP-4 to activate and that of LDN214117 to block SMAD-dependent BMP signaling was also observed on ID1, a BMP target gene. A steady increase of ID1 protein starting from 30 min up to 3 and 24 h after stimulation of the investigated lung cancer cell line with 50 and 100 ng/ml BMP-4 (Fig. 1a, c). The effect was completely diminished 3 h after LDN-214117 incubation of LCLC-103H cells. A 30-min pre-treatment with LDN-214117 before BMP-4 incubation was not as efficient in blocking ID1 production, suggesting a time-dependent effect of LDN-214117 activity on gene regulation level and/or a BMP signaling pathway other than SMAD-dependent that is responsible for ID1 targeting.
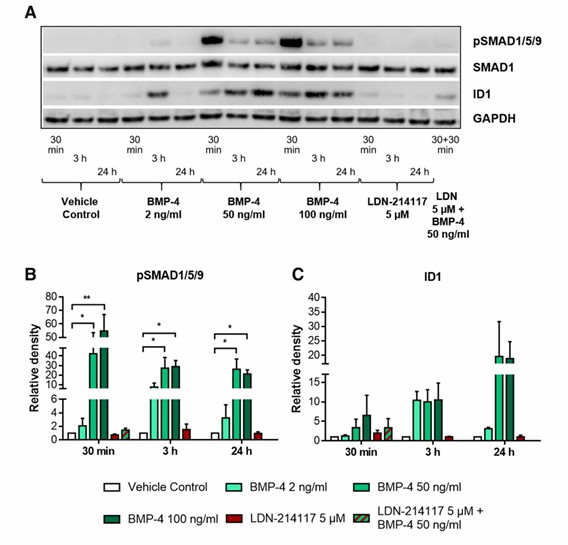 Fig. 1 BMP-4 activates SMAD-dependent BMP signaling pathway in LCLC-103H cells via phosphorylation of SMAD1/5/9. (Mihajlović J, et al., 2019)
Fig. 1 BMP-4 activates SMAD-dependent BMP signaling pathway in LCLC-103H cells via phosphorylation of SMAD1/5/9. (Mihajlović J, et al., 2019)
Ionomycin Induces Apoptosis in LCLC-103H Cells
Ubiquitous calpains (μ- and m-calpain) have been repeatedly implicated in apoptosis. In the course of calpain localization studies, LCLC-103H cells overexpressing the catalytic subunit of μ-calpain rapidly underwent cell death after treatment with ionomycin. To monitor this process in cells not overexpressing calpain, LCLC-103H cells were labeled transiently with GFP or stably with a histone-ECFP chimera. The first signs of ionomycin-induced cell death were detected 3 h after the addition of the ionophore. Typical hallmarks of apoptosis, including cell blebbing and fragmentation, were prevented for 24 h by preincubation with the selective calpain inhibitor AC27P (Fig. 2A and B). Untreated cells overexpressing GFP (Fig. 2C) or the chimeric-ECFP protein were not impaired in their vitality, ruling out that ectopic expression of either protein is cytotoxic for this cell line.
The addition of 2 μm ionomycin to LCLC-103H cells caused an instantaneous increase in intracellular Ca2+ concentration from 50 to 180 nm (Fig. 2D). Remarkably, calcium concentrations were raised transiently to 0.8-1.5 μm. Concomitantly, calpains were activated, as reflected by almost a double increase of Suc-LLVY-amc cleavage inhibited by AC27P (Fig. 2E). In addition, nuclear condensation was detected in the histone-ECFP-labeled cells 3 h after addition of ionomycin (Fig. 3A), and it was inhibited by preincubation with AC27P (Fig. 3C). DNA and protein analysis in ionomycin-treated cultures revealed DNA fragmentation (Fig. 3D) and PARP cleavage to an 85-kDa fragment typical of caspase-mediated apoptosis (Fig. 3F). The same observations were made in etoposide-treated LCLC-103H cells (Fig. 3B) and COS 7 cells after ionomycin addition (Fig. 3E), suggesting an apoptotic process, although its onset was delayed in comparison to ionomycin-treated LCLC-103H cells. These findings demonstrate that ionomycin induces apoptosis in these cells in a calpain-dependent manner.
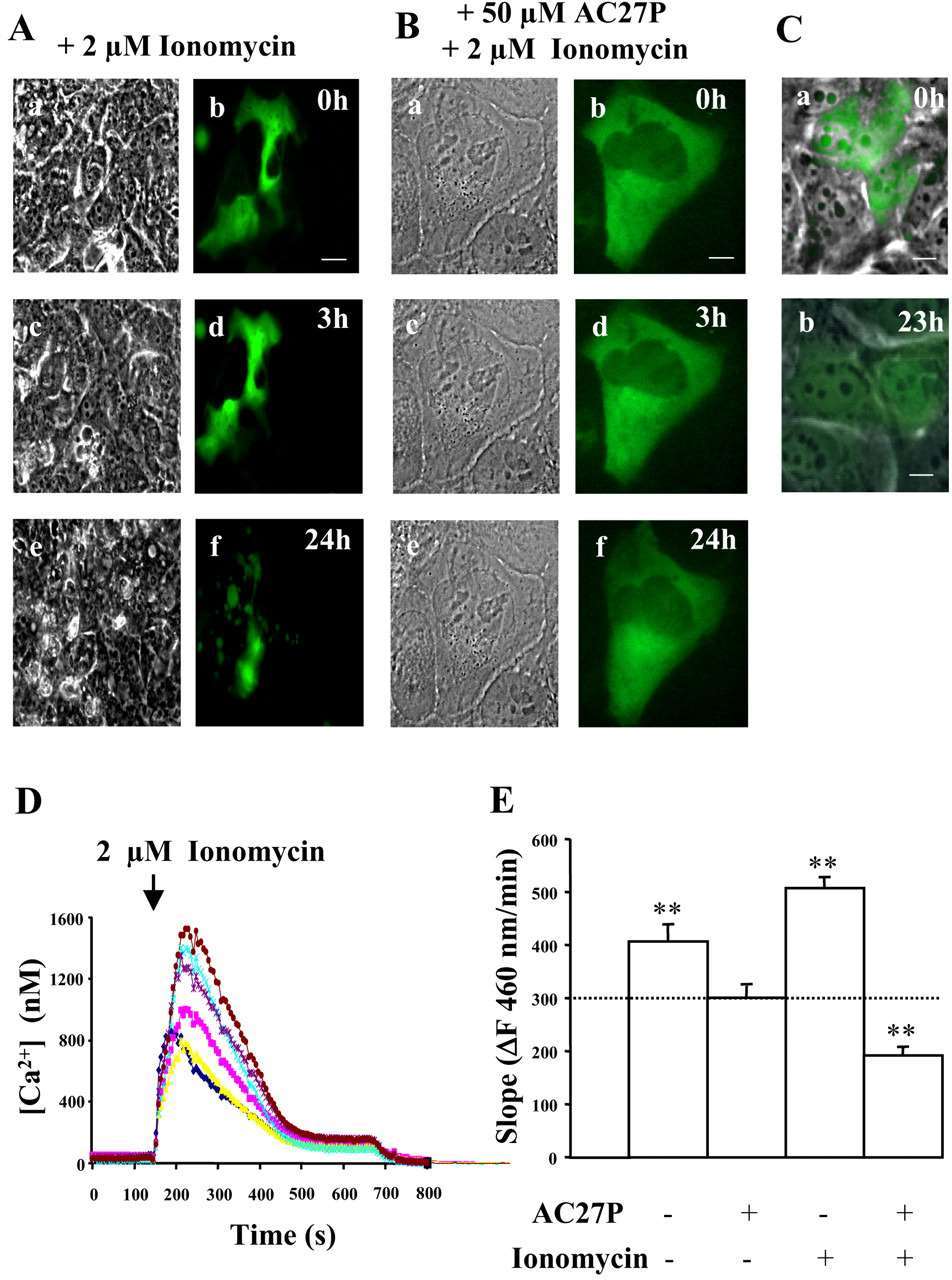 Fig. 2 Induction of cell death and calpain activation in LCLC-103H cells by ionomycin. (Gil-Parrado S, et al., 2002)
Fig. 2 Induction of cell death and calpain activation in LCLC-103H cells by ionomycin. (Gil-Parrado S, et al., 2002)
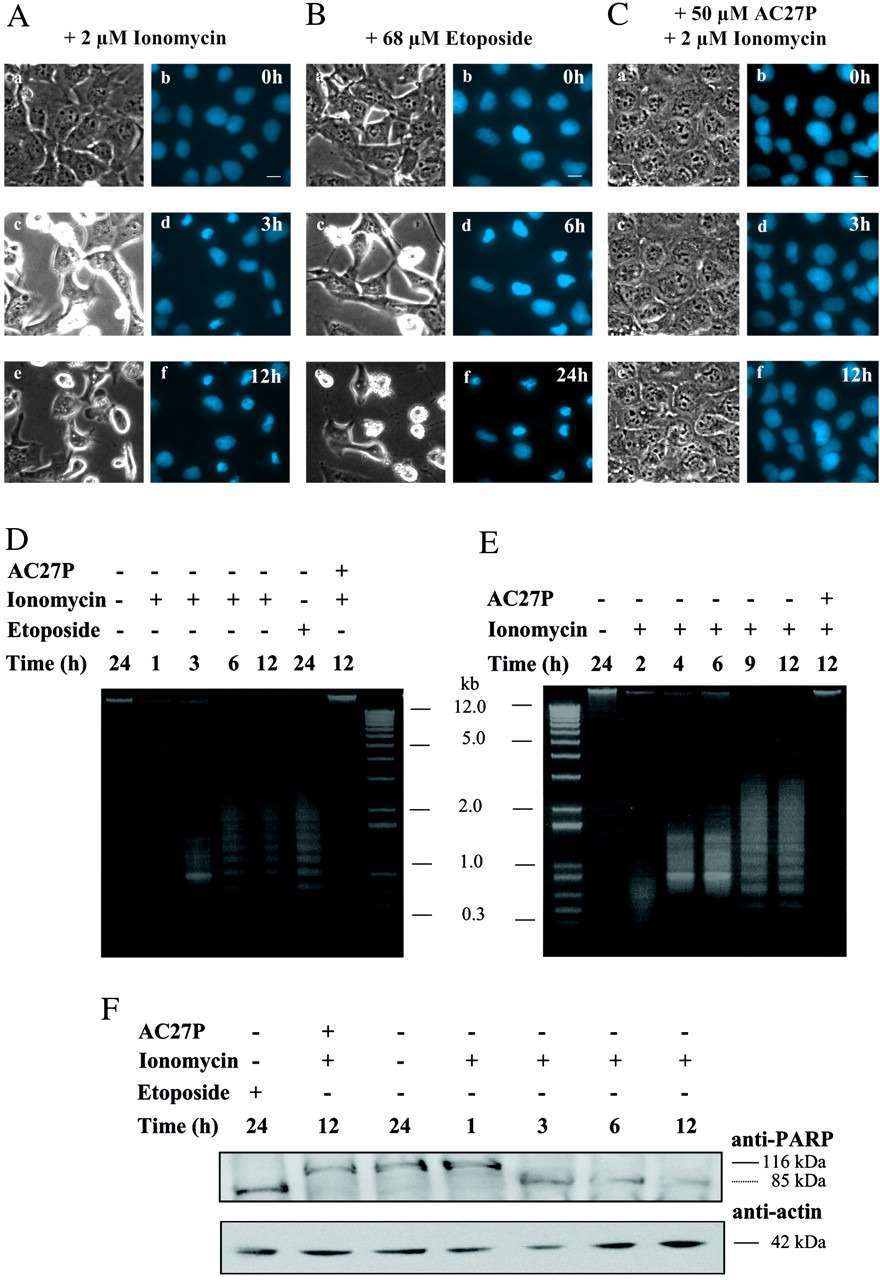 Fig. 3 Hallmarks of apoptosis in ionomycin-treated LCLC-103H cells. (Gil-Parrado S, et al., 2002)
Fig. 3 Hallmarks of apoptosis in ionomycin-treated LCLC-103H cells. (Gil-Parrado S, et al., 2002)
Cell growth refers to the increase in cell size (mass accumulation) while cell division describes the division of a mother cell into two daughter cells (1->2->4->8, etc.). Cell proliferation is the process of generating an increased number of cells through cell division.
Ask a Question
Average Rating: 4.0 | 1 Scientist has reviewed this product
Exceptional reliablity
The reliability of this product is exceptional.
19 Mar 2023
Ease of use
After sales services
Value for money
Write your own review
- You May Also Need