Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY

GOS-3
Cat.No.: CSC-C0452
Species: Human
Source: glioblastoma
Morphology: fibroblastoid, adherent cells growing as monolayer
Culture Properties: monolayer
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Immunology: cytokeratin -, desmin -, endothel -, GFAP +, neurofilament -, vimentin +
Viruses: ELISA: rev
GOS-3 cells were originally described as a glioma cell line isolated from the brain tumour of a 55-year-old male with mixed astrocytoma (grade II/III). Later short tandem repeat (STR) analysis showed that the GOS-3 cells themselves were a product of the human glioblastoma cell line U-343-MG. These cells are widely used in scientific experiments to simulate the biological features of human glioblastoma. GOS-3 cells look like fibroblasts in morphology with their spindle-shaped structure, oval nuclei, abundant cytoplasm and an adherent monolayer growth. On a molecular level, GOS-3 cells carry glial fibrillary acidic protein (GFAP), vimentin, S-100 protein, pp60C-SRC, ciliary neurotrophic factor (CNTF), leukemia inhibitory factor (LIF) and their receptors. The key is that, as seen by experimental evidence, both LIF and oncostatin M in this cell line have the ability to suppress cell growth and differentiation, which suggests that they are important regulators of cell proliferation and differentiation.
Because of their glioma origin, scientists can leverage the GOS-3 cell line to learn about how glioma grows, invades and spreads, and to seek targets for treatment. Additionally, the cell line can be used to test anti-tumor compounds, which could help create new therapies. In addition, GOS-3 cells offer an ideal tool for studying fundamental biological processes including cell proliferation, differentiation, apoptosis and signal transduction.
ALDH5A1 Gene Knockdown Reduces Glioblastoma Cell Viability
Gliomas are the most frequent primary malignant brain tumours. Metabolic reprogramming – the malfunctioning of ALDH enzymes – contributes to glioma development. Piperi et al. investigates the biology and clinical relevance of ALDH5A1, a lesser-studied member of the ALDH family, in diffuse gliomas. In order to explore ALDH5A1's function in glioma growth, they siRNA knocked ALDH5A1 out of the highly expressed glioblastoma cell lines GOS-3 and SJ-GBM2. The western blot analysis confirmed ALDH5A1 silence by a 61% reduction in SJ-GBM2 cells and 49% in GOS-3 cells after 48 hours of transfection (Fig. 1A). 24 hour XTT analyses indicated reduced cell viability in siALDH5A1-transfected cells relative to controls, 53.1% lower in SJ-GBM2 and 38.2% lower in GOS-3 (Fig. 1B). BAX mRNA levels rose but were not statistically significant (Fig. 1C). During ALDH5A1 knockdown, both cell lines demonstrated increased caspase-3 cleavage (Fig. 1D). These results show that ALDH5A1 down-regulation inhibits glioma cell survival and induces apoptosis.
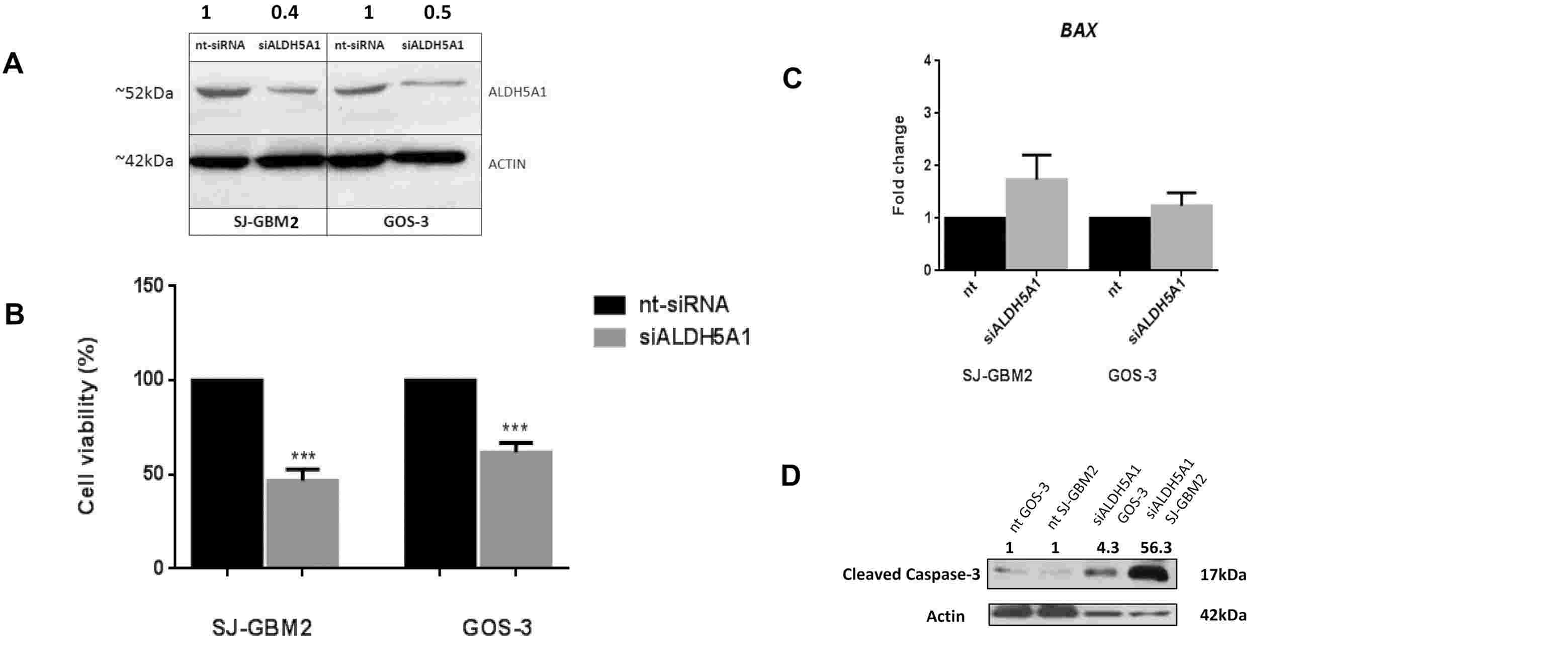 Fig. 1. Glioblastoma cells exhibit reduced cell proliferation following siALDH5A1 transfection, increased BAX mRNA levels and induced caspase-3 cleavage (Piperi, C., Saurty-Seerunghen, M. S., et al., 2023).
Fig. 1. Glioblastoma cells exhibit reduced cell proliferation following siALDH5A1 transfection, increased BAX mRNA levels and induced caspase-3 cleavage (Piperi, C., Saurty-Seerunghen, M. S., et al., 2023).
PCDHB4 Inhibits Cell Proliferation and Migration
Mutant DNA methylation regulates gene expression in GBM, but not PCDHB4 in GBM. Huang et al. discovered a new methylation-dependent gene, PCDHB4, for the diagnosis and prognosis of GBM and shown that PCDHB4 was a tumour suppressor in vitro. The qRT-PCR data indicated that the mRNA expression of PCDHB4 was markedly lower in GBM tumor tissue than in surrounding normal tissue (Fig. 2A). Moreover, 5-Aza treatment of hypermethylated GOS3 and U87MG cell lines led to increased expression of PCDHB4 mRNA at 1 M and 2 M concentrations, respectively (Fig. 2B and C). To investigate whether PCDHB4 was a methylated gene in GBM, they correlated PCDHB4 methylation with mRNA levels in GOS3 and U87MG cell lines. They constructed and verified PCDHB4 overexpression plasmids and analyzed cell proliferation and vitality (Fig. 2D and E). Overexpression of PCDHB4 reduced cell proliferation and vitality, as shown in CCK8 (Fig. 2F and G) and EdU assays (Fig. 3A and B). Additionally, wound healing assays indicated significantly reduced migration capabilities in PCDHB4 over-expressing cells (Fig. 3C and D). These findings suggest PCDHB4 inhibits GBM progression.
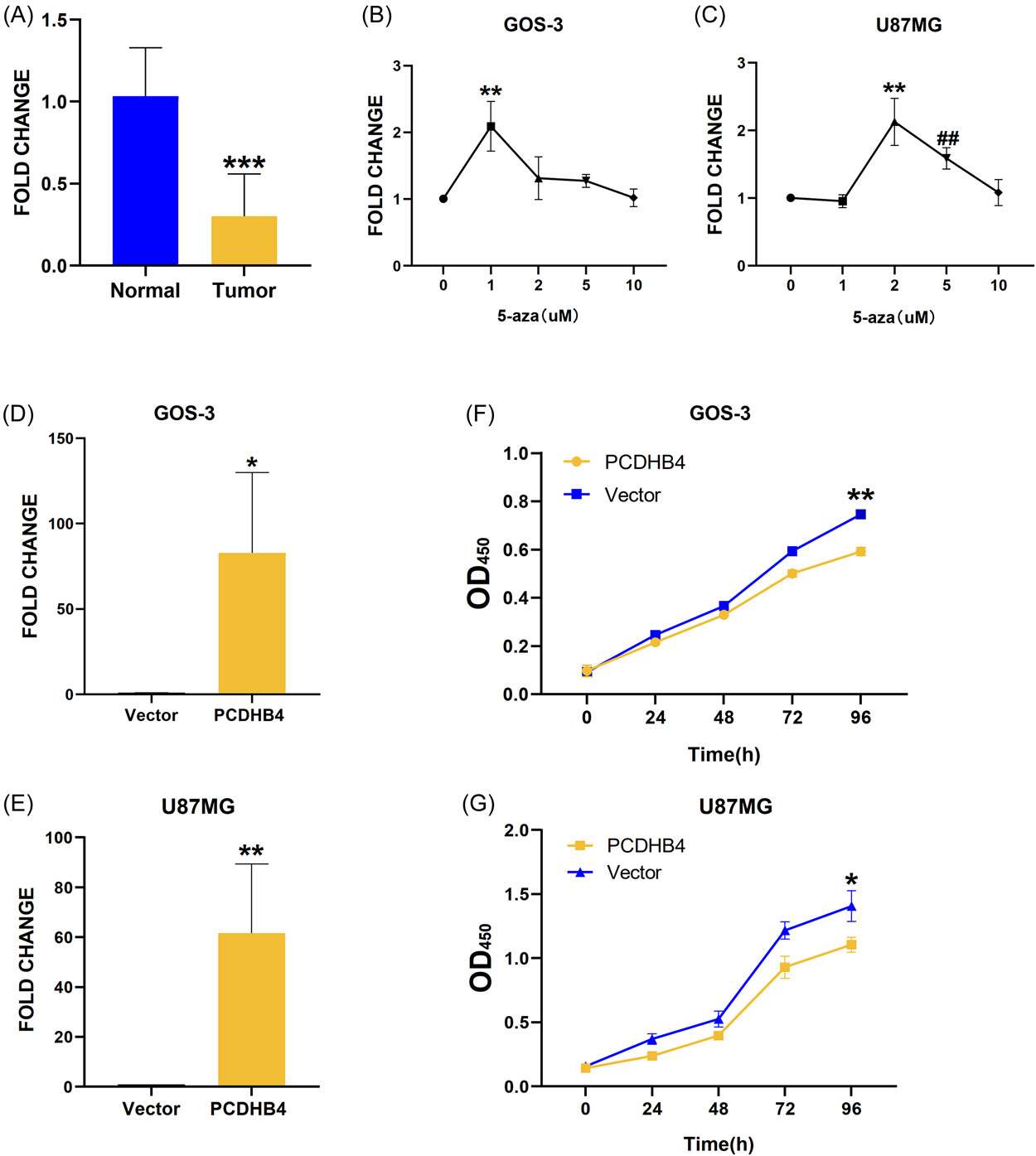 Fig. 2. PCDHB4 downregulated in glioma tissues and increased with demethylation (Huang, H., Xie, Y., et al., 2023).
Fig. 2. PCDHB4 downregulated in glioma tissues and increased with demethylation (Huang, H., Xie, Y., et al., 2023).
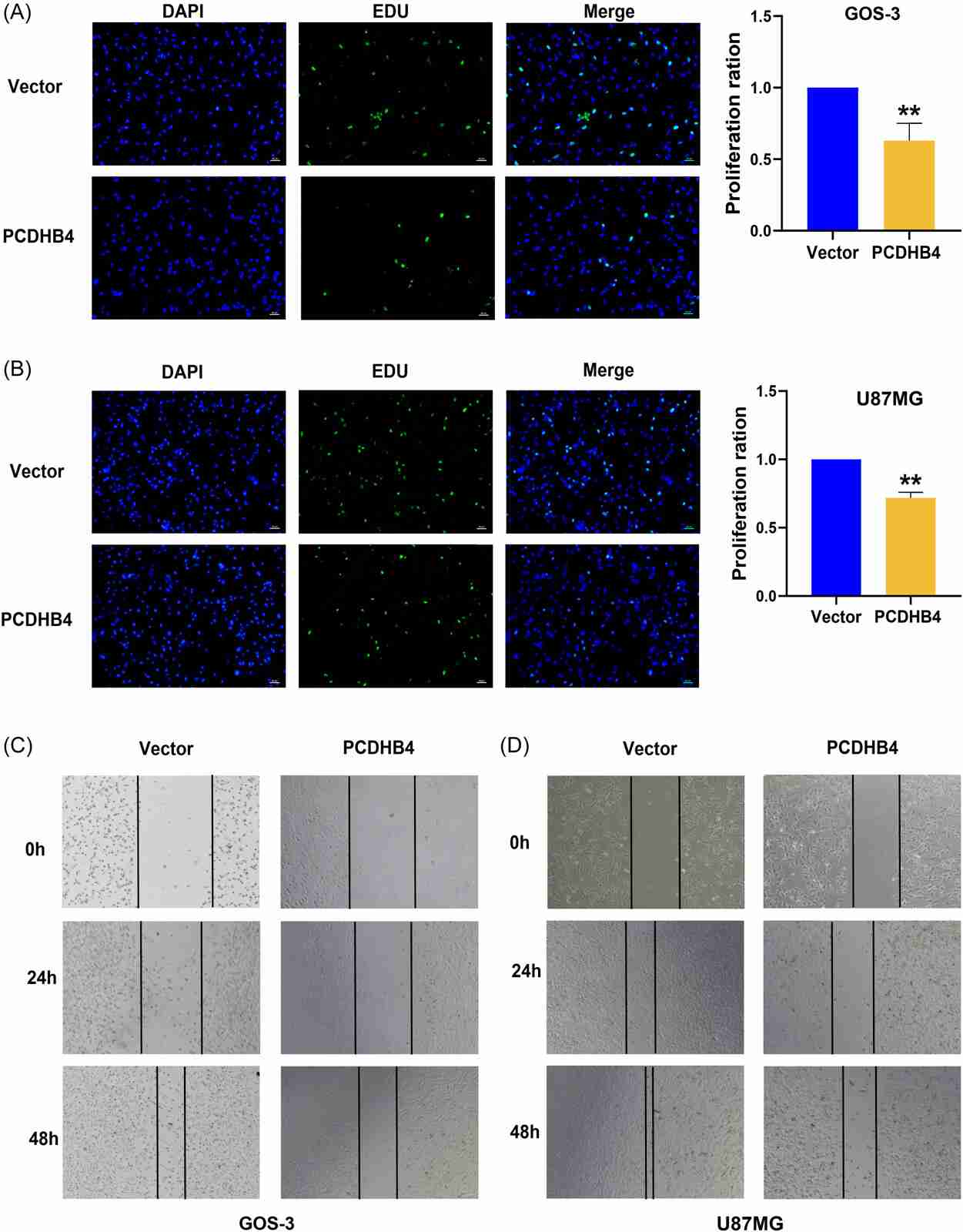 Fig. 3. PCDHB4 inhibits cell proliferation and migration (Huang, H., Xie, Y., et al., 2023).
Fig. 3. PCDHB4 inhibits cell proliferation and migration (Huang, H., Xie, Y., et al., 2023).
Collect the cells by centrifugation, remove the supernatant thoroughly from the centrifuge tube, add the appropriate amount of serum-free cell lyophilisation solution to the tube to make a cell lyophilisation suspension and place in a -80°C refrigerator for long-term freezing or transfer to liquid nitrogen
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
Ideal products
This product is ideal for studying cell migration due to cell-cell interaction with the extracellular matrix.
05 Oct 2021
Ease of use
After sales services
Value for money
Write your own review
- You May Also Need