Unveiling the Molecular Secrets of Adipogenesis in MSCs
Mesenchymal stem cells (MSCs) are multipotent stromal cells that can differentiate into adipogenic, osteogenic, chondrogenic, and other lineages. MSCs derived from many tissues, such as bone marrow, umbilical cord, placenta, skin, and fat. MSCs have attracted increasing interest in regenerative medicine for easy isolation, rapid proliferation, low immunogenicity, and multilineage differentiation potential. Adipogenic differentiation is an important direction of MSC differentiation for it plays an important role in soft tissue defect repair in traumatology. Moreover, excessive adipogenic differentiation of MSCs is related to skeletal diseases such as osteoporosis and osteonecrosis.
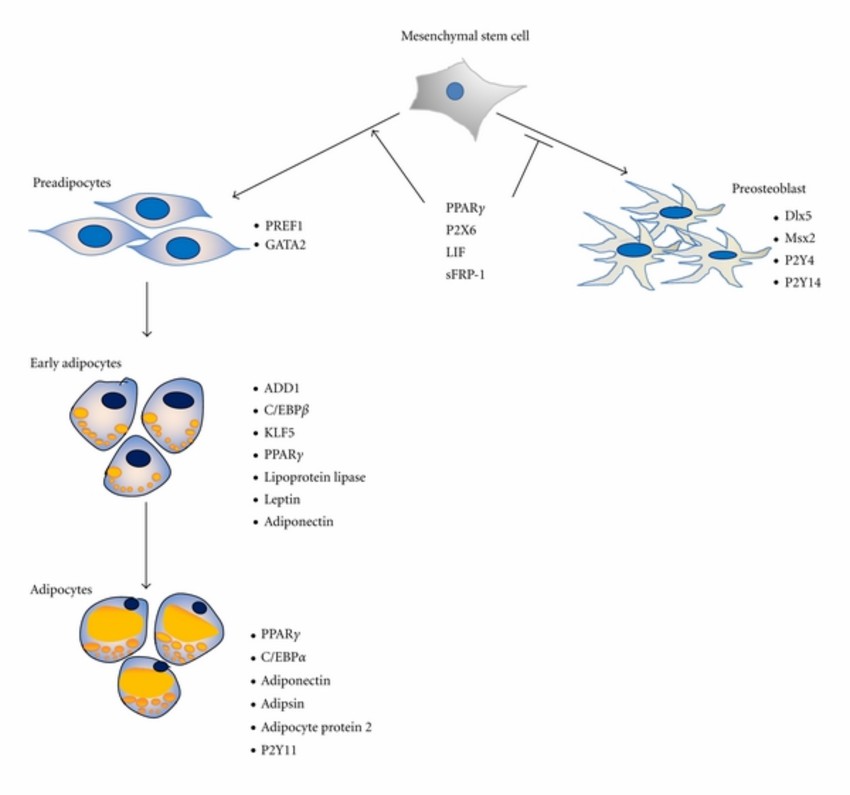 Fig.1 Differentiation of MSCs towards adipocytes. (Zhang Y, et al., 2012)
Fig.1 Differentiation of MSCs towards adipocytes. (Zhang Y, et al., 2012)
What Is Adipogenic Differentiation and Dedifferentiation of MSCs?
During adipogenic differentiation, MSCs undergo a remarkable transformation, transitioning from a multipotent state to becoming committed preadipocytes and ultimately mature, lipid-laden adipocytes. This intricate process is tightly regulated by a complex interplay of transcription factors, signaling cascades, and epigenetic modifications.
Interestingly, the process of adipogenic differentiation is not irreversible. MSCs possess the remarkable ability to dedifferentiate, reverting to a more primitive, multipotent state. This dedifferentiation process is equally intriguing, as it allows MSCs to regain their plasticity and potential for multilineage differentiation, opening up new avenues for therapeutic applications.
Molecular Mechanisms Involved in Adipogenic Differentiation and Dedifferentiation
At the heart of the adipogenic differentiation process in MSCs lies a well-orchestrated network of microRNAs (miRNAs), transcription factors (TFs), and signaling pathways that work in concert to drive the transformation from a multipotent state to committed preadipocytes and ultimately mature, lipid-laden adipocytes.
| miRNAs | miR-377-3p | miR-377-3p has been identified as a potent negative regulator of the adipogenic differentiation pathway in MSCs. This miRNA exerts its inhibitory influence by directly targeting the key transcription factor PPAR-γ, the master regulator of adipogenesis. By downregulating PPAR-γ expression, miR-377-3p effectively blocks the activation of the adipogenic transcriptional network, thereby suppressing the commitment and maturation of MSCs into adipocytes. |
| miR-431 | In contrast, our studies have revealed that miR-431 plays a pivotal role in the dedifferentiation of mature adipocytes. This miRNA acts by targeting the transcription factor FOXN1, which is crucial for the maintenance of the adipocyte phenotype. By downregulating FOXN1, miR-431 enables the reversion of adipocytes to a more primitive, multipotent state, allowing them to regain their differentiation potential and expand the therapeutic possibilities of MSC-based interventions. | |
| TFs | FOXN1 | The FOXN1 has emerged as a key player in the maintenance of the mature adipocyte phenotype. This forkhead box protein acts as a positive regulator of adipocyte-specific genes, ensuring the sustained expression of markers such as FABP4 and adiponectin. The downregulation of FOXN1, as facilitated by miR-431, is a crucial step in the dedifferentiation of adipocytes, enabling the reacquisition of multipotency. |
| TEAD4 | TEAD4, a member of the TEA domain family, has been shown to play a pivotal role in the early stages of adipogenic differentiation, where it collaborates with the master regulator PPAR-γ to drive the activation of adipocyte-specific genes. The intricate interplay between TEAD4 and PPAR-γ is a critical node in the adipogenic transcriptional network, making it a promising target for therapeutic interventions. | |
| Signaling pathways | PPARγ pathway | At the heart of the adipogenic differentiation pathway lies the peroxisome proliferator-activated receptor gamma (PPARγ) signaling axis. As the master regulator of adipogenesis, PPARγ acts as a transcriptional activator, driving the expression of adipocyte-specific genes and enabling the commitment and maturation of MSCs into functional adipocytes. The delicate balance of PPARγ signaling is a key determinant in the fate of MSCs during the adipogenic differentiation process. |
| Wnt/β-catenin pathway | The Wnt/β-catenin signaling pathway has been identified as a negative regulator of adipogenic differentiation. The activation of this pathway promotes the maintenance of the multipotent state of MSCs, inhibiting the expression of PPAR-γ and C/EBPs, the master regulators of the adipogenic program. The interplay between Wnt/β-catenin signaling and the PPARγ pathway serves as a critical checkpoint in the commitment of MSCs to the adipogenic lineage. | |
| Transforming growth factor-β pathway | The transforming growth factor-β (TGF-β) signaling pathway has emerged as a key player in the dedifferentiation of mature adipocytes. By activating this pathway, the experts have observed the reversion of adipocytes to a more primitive, multipotent state, enabling them to regain their capacity for multilineage differentiation. | |
| Notch signaling pathway | The Notch signaling pathway has also been implicated in the fine-tuning of the adipogenic differentiation process. This pathway can exert both positive and negative regulatory effects, depending on the specific context and the interplay with other signaling cascades. |
Creative Bioarray Relevant Recommendations
| Product Types | Description |
| SuperCult® Mesenchymal Stem Cell Adipogenic Differentiation Medium Kit | Creative Bioarray's mesenchymal stem cell media were developed for the in vitro expansion and directed differentiation of MSC from bone marrow, the umbilical cord matrix (Wharton's Jelly), and adipose tissue. |
| SuperCult® Human Mesenchymal Stem Cell Adipogenic Differentiation Medium | SuperCult® Human Mesenchymal Stem Cell Adipogenic Differentiation Medium Kit provides both an induction medium and a maintenance medium guaranteed to induce adipogenic differentiation of human bone marrow-derived MSCs into mature, functionally active adipocytes. |
| SuperCult® Murine Mesenchymal Stem Cell Adipogenic Differentiation Medium Kit | SuperCult® Murine Mesenchymal Stem Cell Adipogenic Differentiation Medium Kit allows for the study of adipogenesis starting from rat MSCs. Adipogenic differentiation can be assayed after 12-14 days in culture. |
Reference
- Zhang Y, et al. (2012). "Mechanisms underlying the osteo- and adipo-differentiation of human mesenchymal stem cells." Scientific World Journal. 793823.