Generation of Immortalized Human Lacrimal Gland Cell Lines
Cells. 2024 Apr 3; 13 (7): 622.
Authors: Gleixner S, Zahn I, Dietrich J, Singh S, Drobny A, Schneider Y, Schwendner R, Socher E, Blavet N, Bräuer L, Gostian AO, Balk M, Schulze-Tanzil G, Günther C, Paulsen F, Arnold P.
INTRODUCTION
The lacrimal gland plays a crucial role in maintaining the health of the ocular surface by secreting the aqueous component of the tear film. Its secreted fluid cleanses the conjunctival sac, moistens the ocular surface, nourishes the cornea, improves optical properties, and plays an important role in immune defense. In the lacrimal gland, specialized epithelial cells (acinar cells) produce and secrete the aqueous component of the tear film, and their dysfunction is directly related to the hyposecretory form of dry eye disease. However, research on the human lacrimal gland is limited due to the lack of primary tissue and the absence of a human lacrimal gland epithelial cell line.
METHODS
Primary human lacrimal gland tissue was obtained after receiving signed consent from an adult female patient by surgical resection at a tertiary referral medical center and was approved by the institutional review board.
- Seeding the minced tissue on a murine fibroblast feeder layer resulted in primary cell outgrowth. After ten days, the murine feeder layer was removed with a trypsin concentration that would not detach the outgrowing more adhesive lacrimal gland cells. In the second step, these cells were then detached with a higher concentrated trypsin solution and harvested as a pool, containing all cell types of the lacrimal gland.
- To obtain a full picture of gene expression for the three SV40-positive cell clones, total RNA sequencing (RNAseq) was performed for four replicates of each cell clone and compared to human lacrimal gland tissue from four donors.
- Browse our recommendations
| Product/Service Types | Description |
| Immortalized Cell Lines | With years of experience in cell immortalization, Creative Bioarray has developed the most comprehensive immortalized cell lines comprising human-immortalized cells, mouse-immortalized cells, and other immortalized cells. |
| Cell Immortalization kit | To surpass senescence, Creative Bioarray has developed several Cell Immortalization Kit for immortalizing mammalian cells in culture. |
| Cell Immortalization Service | Creative Bioarray is offering cell immortalization services. Based on our experienced scientist team and elaborate technical platforms, we have been able to successfully immortalize cells from any species and any tissue with the function you need. |
RESULTS
- A SV40-carrying plasmid was then introduced into the cells by electroporation. After an additional proliferation period, individual cell clones were selected from the pool for further characterization (Fig. 1A). Reverse transcription polymerase chain reaction (RT-PCR) was performed for different marker genes to select clones that showed the expression of epithelial cells. For comparative reasons, a human corneal epithelial cell line (CEC), human mesenchymal stem cells (hMSC), and a fibroblast line (3T3 fibro) were included (Fig.1B). Markers for epithelial, mesenchymal and endothelial cells and fibroblasts (Fig. 1C) and identified clones 1, 3 and 6 to express E-cadherin (CADH1), sialomucine (CD34), smooth muscle actin (ACTA2), vimentin (VIM) and the two transcription factors Forkhead Box C1 (FOXC1) and Paired Box Gene 6 (PAX6) were included, which are important for the differentiation of cells in various types of ocular epithelium (Fig.1B, C). Compared to clones 1, 3, and 6, there was a marked difference in gene expression for clone 5, which showed similarity to the expression pattern of the fibroblast control line (strong for ACTA2 and VIM). Thus, clone 5 was utilized as a fibroblast control for the next experiments. Then, different clones were tested for the expression of the inserted SV40 gene by RT-PCR (Figure 1D). Clones 1, 3, and 6, the lacrimal gland cell pool, and the CEC line were positive for SV40, whereas 3T3 fibroblasts, hMSC, clone 5 fibroblasts, and the H2O control showed no signal for SV40 (Fig. 1D). To demonstrate the localization of the SV40 gene product in the nucleus, clones 1, 3, and 6 for SV40 were stained and clone 5 was included as a negative control. To visualize the cell structure, F-actin was included (Fig. 1E).
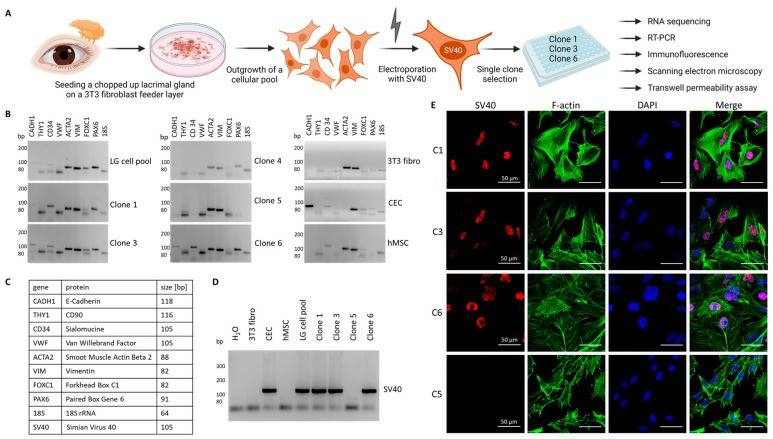 Fig. 1 Selection of immortalized human lacrimal gland cells.
Fig. 1 Selection of immortalized human lacrimal gland cells.
- A differential expression analysis was performed and found similar genes within the top 20 up- or downregulated genes in all three cell clones (Fig. 2A). Metallothionein 2A (MT2A), DNA topoisomerase II alpha (TOP2A) and two different genes for structural maintenance of chromosomes (SMCs), SMC2 and SMC4, were upregulated in all three cell clones, whereas four ribosomal protein (RPL) genes (RPL3, 7, 10, 21), eukaryotic translation elongation factor 1 alpha 1 (EEF1A1) and SRP receptor subunit alpha (SRPRA) were downregulated. To identify regulated pathways, the top 100 up- and downregulated genes for each clone were analyzed using HumanMine's (humanmine.org) GOterm gene analysis tool. Similar pathways significantly upregulated in all three cell clones were identified, mostly related to cell proliferation, cell division, and chromosome segregation (Fig. 2B). Among the downregulated GOterms, it is identified that mainly those associated with cellular, structural, or tissue homeostasis (Fig. 2B). Next, the expression levels of marker genes were compared for epithelial cells, endothelial cells, hMSC, myoepithelial cells, and fibroblasts between the cell clones and total lacrimal gland tissue (Fig. 2C).
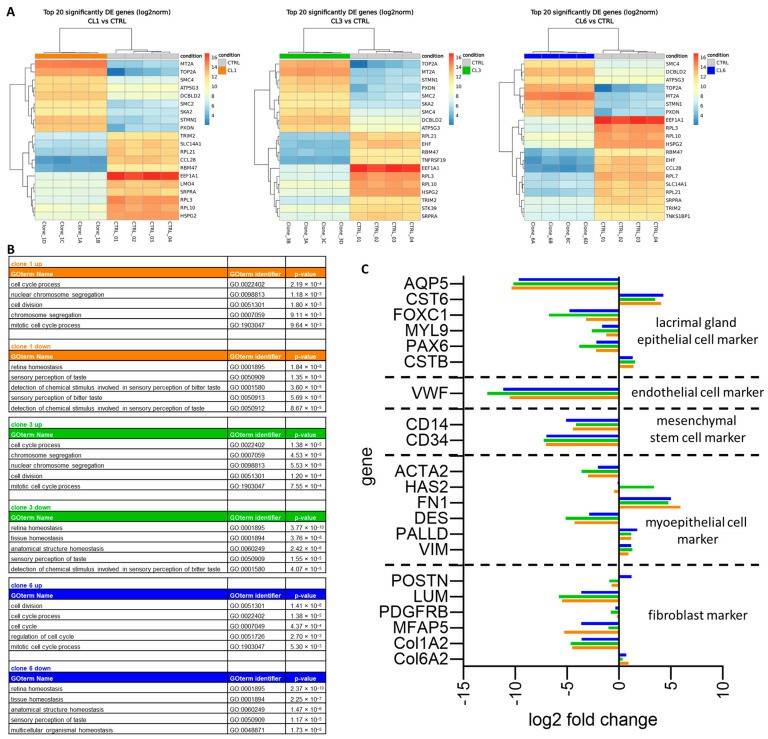 Fig. 2 Total RNA sequencing of immortalized human lacrimal gland cells.
Fig. 2 Total RNA sequencing of immortalized human lacrimal gland cells.
SUMMARY
Here, the generation of immortalized human lacrimal gland cell lines through the introduction of an SV40 antigen. Three cell clones were successfully isolated and characterized from a female lacrimal gland donor, obtaining total RNAseq.
RELATED PRODUCTS & SERVICES
Reference
- Gleixner S, et al. (2024). "A New Immortalized Human Lacrimal Gland Cell Line." Cells. 13 (7): 622.