Establishment of Cell Lines Using a Non-viral Immortalization Approach
Biological Research. 2024 May 4; 57 (1): 21.
Authors: Lange S, Kuntze A, Wüstmann N, Reckers T, Humberg V, Dirks WG, Huss S, Vieler J, Schrader AJ, Bögemann M, Schlack K, Bernemann C.
INTRODUCTION
Research on prostate cancer is mostly performed using cell lines derived from metastatic disease, not reflecting stages of tumor initiation or early progression. The establishment of cancer cell lines derived from the primary tumor site has not been described so far. By definition, cancer cells can be cultured indefinitely, whereas normal epithelial cells undergo senescence in vitro. Epithelial cells can be immortalized, accomplished by using viral integration of immortalization factors. Viral approaches, however, might be impaired by regulatory and safety issues as well as random integration into regulatory genetic elements, modifying precise gene expression. Surgical specimens of prostate cancer patients were performed non-viral, Sleeping Beauty (SB) transposase-based immortalization of prostate epithelial cells.
METHODS
- Primary epithelial cell cultures were electroporated at about passage 4-7 using different immortalization factor combinations, i.e., SV40LT only (MS-pPC-184S, MS-pPC-191S, MS-pPC-192S; MS-pPC-193S), hTERT only (MS-pPC-184 T) and SV40LT and hTERT (MS-pPC-184ST, MS-pPC-191ST, MS-pPC-193ST).
- For authentication, primary cell cultures and immortalized cell lines were analyzed for consistency with patient tumor tissue. Therefore, short tandem repeat (STR) analyses on both, cell lines and matching tumor tissues were performed.
- Browse our recommendations
| Product/Service Types | Description |
| Prostate Cells | Creative Bioarray can offer six types of human primary prostate cells to meet your different research requirements. |
| Prostate Tumor Cells | Creative Bioarray provides human prostate tumor cells that have the original pathological diagnosis and are analyzed for key mutations. |
| Cell Immortalization Service | Creative Bioarray is offering cell immortalization services. Based on our experienced scientist team and elaborate technical platforms, we have been able to successfully immortalize cells from any species and any tissue with the function you need. |
RESULTS
- MS-pPC-184S, -T, and -ST cells displayed senescence within passages 15, 15, and 20, respectively, demonstrating no successful immortalization of MS-pPC-184 specimen. MS-pPC-191S showed senescence at passage 45, whereas -191ST could be passaged further (> 100 passages). Furthermore, MS-pPC-192S, MS-pPC-193S, and -193ST showed no signs of senescence. These four cell cultures (MS-pPC-191ST, MS-pPC-192S, MS-pPC-193S, and MS-pPC-193ST) acquired common characteristics of cell lines, i.e., unique cell morphology and growth characteristics (Fig. 1A). Stable integration was determined by PCR on gDNA of electroporated cells. Amplicons span the immortalization cassette including integration factors hTERT or SV40LT, EF1α promotor, RPBSA promotor, and RFP or GFP, respectively (Fig. 1B). Additionally, immortalized cell lines showed both SV40LT and hTERT expression, whereas parental cell cultures were negative for both factors (Fig. 1C, D). These results indicate a successful immortalization of primary prostatic epithelial cells into four different cell lines.
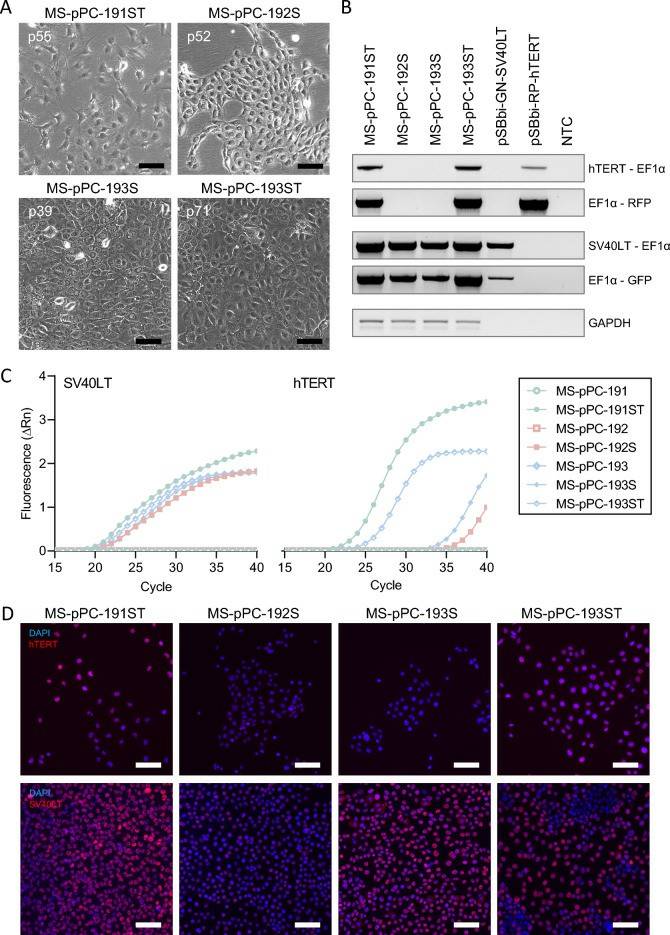 Fig. 1 Immortalization of cell lines by stable integration.
Fig. 1 Immortalization of cell lines by stable integration.
- All cell lines showed identical STR profiles compared to patients' tumor tissues. Furthermore, none of the primary cell lines showed contamination with established cell lines used in the laboratory. Thus, the successful establishment of cell lines derived from matched patient samples. Next, the expression of epithelial prostate (cancer) markers KRT, AR, and EpCAM (Fig. 2A). KRT and EpCAM expression was detected in MS-pPC-192S, 193S, and 193ST, whereas 191ST were negative for both markers. Additionally, none of these cell lines displayed expression of AR. Benign prostate epithelial cell line RWPE-1 showed a similar expression pattern, with KRT and EpCAM, but no AR expression (Fig. 2B). In contrast, LNCaP—a metastasis-derived prostate cancer cell line—showed expression of all markers. These results suggest an epithelial, yet not prostate cancer-related origin of cell lines MS-pPC-192S, 193S, and 193ST. When analyzing AR activity in response to hormonal induction using luciferase assays, a drastic increase in AR activity in LNCaP cells, whereas no changes in activity were observed in newly derived cell lines (Fig. 2C).
- Thereafter, flow cytometry analysis was performed for expression of basal (CD49f) and luminal (CD26) markers (Fig. 2D). No cell line displayed a sole luminal expression pattern but either a mixture of both markers or basal marker only. Subsequently, RNAseq analysis of four cell lines was performed along with prostate (cancer) cell lines RWPE-1, LNCaP, and PC-3. Principal component analysis (PCA) demonstrated the closest proximity of MS-pPC-193S, -ST, and -192S to the RWPE-1 cell line (Fig. 2E). MS-pPC-191ST showed less proximity, although closer to RWPE-1 than any other reference cell line. Prostate cancer metastasis-derived cell lines PC-3 and LNCaP cell lines showed a high distance to immortalized cell lines. These results suggest that immortalized cell lines reflect healthy prostate cells rather than metastasis-derived prostate cancer cells.
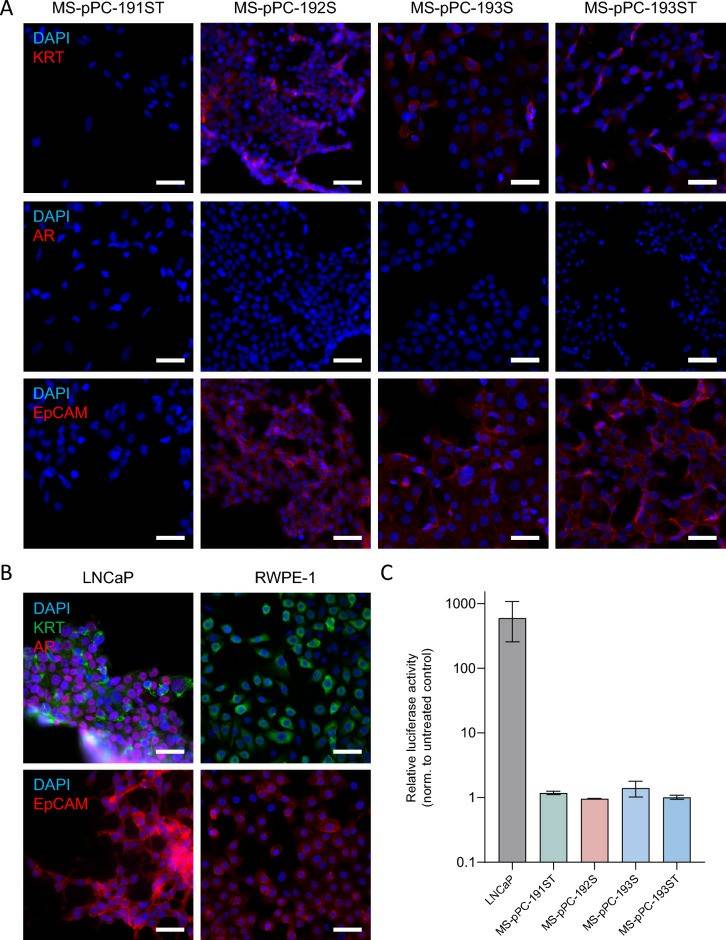 Fig. 2 Characterization of newly established cell lines.
Fig. 2 Characterization of newly established cell lines.
SUMMARY
By using SB transposase-based integration of immortalization factors, primary prostate cell lines were established. Three out of four cell lines displayed epithelial characteristics, however without expression of prostate (cancer) characteristics.
RELATED PRODUCTS & SERVICES
Reference
- Lange S, et al. (2024). "Establishment of primary prostate epithelial and tumorigenic cell lines using a non-viral immortalization approach." Biol Res. 57 (1): 21.