- You are here: Home
- Resources
- Life Science Articles
- Diverse Biological Functions of Extracellular Vesicles
Resources
-
Cell Services
- Cell Line Authentication
- Cell Surface Marker Validation Service
-
Cell Line Testing and Assays
- Toxicology Assay
- Drug-Resistant Cell Models
- Cell Viability Assays
- Cell Proliferation Assays
- Cell Migration Assays
- Soft Agar Colony Formation Assay Service
- SRB Assay
- Cell Apoptosis Assays
- Cell Cycle Assays
- Cell Angiogenesis Assays
- DNA/RNA Extraction
- Custom Cell & Tissue Lysate Service
- Cellular Phosphorylation Assays
- Stability Testing
- Sterility Testing
- Endotoxin Detection and Removal
- Phagocytosis Assays
- Cell-Based Screening and Profiling Services
- 3D-Based Services
- Custom Cell Services
- Cell-based LNP Evaluation
-
Stem Cell Research
- iPSC Generation
- iPSC Characterization
-
iPSC Differentiation
- Neural Stem Cells Differentiation Service from iPSC
- Astrocyte Differentiation Service from iPSC
- Retinal Pigment Epithelium (RPE) Differentiation Service from iPSC
- Cardiomyocyte Differentiation Service from iPSC
- T Cell, NK Cell Differentiation Service from iPSC
- Hepatocyte Differentiation Service from iPSC
- Beta Cell Differentiation Service from iPSC
- Brain Organoid Differentiation Service from iPSC
- Cardiac Organoid Differentiation Service from iPSC
- Kidney Organoid Differentiation Service from iPSC
- GABAnergic Neuron Differentiation Service from iPSC
- Undifferentiated iPSC Detection
- iPSC Gene Editing
- iPSC Expanding Service
- MSC Services
- Stem Cell Assay Development and Screening
- Cell Immortalization
-
ISH/FISH Services
- In Situ Hybridization (ISH) & RNAscope Service
- Fluorescent In Situ Hybridization
- FISH Probe Design, Synthesis and Testing Service
-
FISH Applications
- Multicolor FISH (M-FISH) Analysis
- Chromosome Analysis of ES and iPS Cells
- RNA FISH in Plant Service
- Mouse Model and PDX Analysis (FISH)
- Cell Transplantation Analysis (FISH)
- In Situ Detection of CAR-T Cells & Oncolytic Viruses
- CAR-T/CAR-NK Target Assessment Service (ISH)
- ImmunoFISH Analysis (FISH+IHC)
- Splice Variant Analysis (FISH)
- Telomere Length Analysis (Q-FISH)
- Telomere Length Analysis (qPCR assay)
- FISH Analysis of Microorganisms
- Neoplasms FISH Analysis
- CARD-FISH for Environmental Microorganisms (FISH)
- FISH Quality Control Services
- QuantiGene Plex Assay
- Circulating Tumor Cell (CTC) FISH
- mtRNA Analysis (FISH)
- In Situ Detection of Chemokines/Cytokines
- In Situ Detection of Virus
- Transgene Mapping (FISH)
- Transgene Mapping (Locus Amplification & Sequencing)
- Stable Cell Line Genetic Stability Testing
- Genetic Stability Testing (Locus Amplification & Sequencing + ddPCR)
- Clonality Analysis Service (FISH)
- Karyotyping (G-banded) Service
- Animal Chromosome Analysis (G-banded) Service
- I-FISH Service
- AAV Biodistribution Analysis (RNA ISH)
- Molecular Karyotyping (aCGH)
- Droplet Digital PCR (ddPCR) Service
- Digital ISH Image Quantification and Statistical Analysis
- SCE (Sister Chromatid Exchange) Analysis
- Biosample Services
- Histology Services
- Exosome Research Services
- In Vitro DMPK Services
-
In Vivo DMPK Services
- Pharmacokinetic and Toxicokinetic
- PK/PD Biomarker Analysis
- Bioavailability and Bioequivalence
- Bioanalytical Package
- Metabolite Profiling and Identification
- In Vivo Toxicity Study
- Mass Balance, Excretion and Expired Air Collection
- Administration Routes and Biofluid Sampling
- Quantitative Tissue Distribution
- Target Tissue Exposure
- In Vivo Blood-Brain-Barrier Assay
- Drug Toxicity Services
Diverse Biological Functions of Extracellular Vesicles
Frontiers in Immunology. 2023; 14: 1120175.
Authors: Karnas E, Dudek P, Zuba-Surma EK.
INTRODUCTION
- In the last few decades, the practical use of stem cells (SCs) in the clinic has attracted significant attention in regenerative medicine due to the ability of these cells to proliferate and differentiate into other cell types. However, recent findings have demonstrated that the therapeutic capacity of SCs may also be mediated by their ability to secrete biologically active factors, including extracellular vehicles (EVs).
- Such submicron circular membrane-enveloped vesicles may be released from the cell surface and harbor bioactive cargo in the form of proteins, lipids, mRNA, miRNA, and other regulatory factors. Notably, growing evidence has indicated that EVs may transfer their bioactive content into recipient cells and greatly modulate their functional fate.
Biological Activity of EVs
 Fig. 1 Biological role of EVs in homeostasis and pathophysiology. (Karnas E, et al., 2023)
Fig. 1 Biological role of EVs in homeostasis and pathophysiology. (Karnas E, et al., 2023)
- Indeed, several studies have demonstrated the role of EVs in the process of information exchange between cells. It has been widely postulated that EVs may contribute to cell-to-cell communication, which includes the step of their interaction with the target cell, that may occur in several ways, by endocytosis, phagocytosis, or by direct fusion with the cell membrane including receptor-ligand interactions, subsequently leading to the release of bioactive cargo.
- EVs can serve as paracrine mediators that target cells by transferring their bioactive content in the form of different types of nucleic acids, receptors, enzymes, transcription factors, immunomodulators, and even morphogenic factors such as Wnt and Notch signaling proteins. Delivery of the EV cargo into the recipient cells opens several ways of potential regulation of cellular processes, including influence on gene and protein expression, as well as activity of intracellular signaling pathways.
- On the other hand, EVs may also participate in the pathogenesis of many diseases. As an example, EVs secreted by tumors may promote their progression by stimulating pro-angiogenic processes and inhibiting the immune system. EVs have also been shown to contribute to the transmission of prions, α-synuclein responsible for the pathogenesis of Parkinson's disease (PD), as well as β-amyloid, which contributes to the development of Alzheimer's disease (AD). Moreover, EVs can transfer the drug resistance phenotype between cells, which is related to the transfer of drug-efflux membrane pumps.
Role of EVs in the Regulation of Immune System
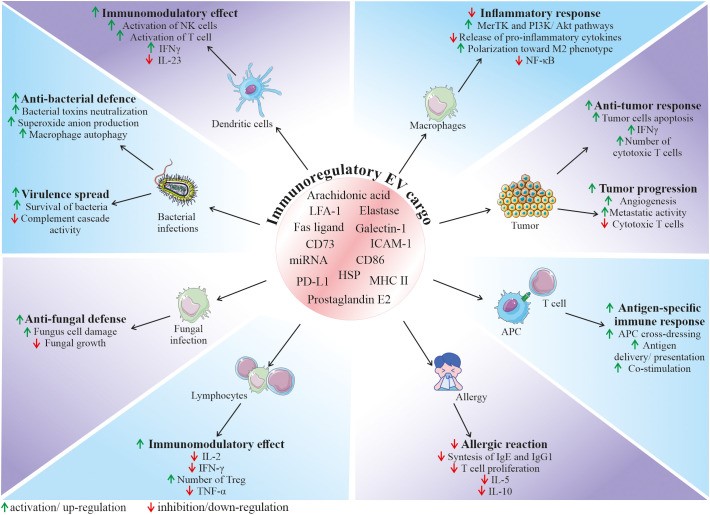 Fig. 2 Role of EVs in the regulation of the immune response. (Karnas E, et al., 2023)
Fig. 2 Role of EVs in the regulation of the immune response. (Karnas E, et al., 2023)
- Among the variety of reported functions, EVs are also envisioned as important factors modulating the function of the immune system, both as activators or inhibitors, depending on the biological context. Their role in immunity relies both on the interaction of EVs from other cell types with immune cells, as well as on the secretion of EVs by the cellular components of the immune system, regulating its fate in the paracrine or autocrine manner. Thus, EVs mediate communication between immune cells, taking part in orchestrating an immune response. In particular, they are a part of the interaction of innate and adaptive immunity, modulating cell response and release of cytokines, chemokines, and other immune-active factors.
- In the context of immune defense against pathogenic factors, EVs are involved in the communication between bacteria and host cells, playing either a protective or pathogenic role in the infection. On one hand, bacteria-derived EVs may serve as shuttle particles contributing to virulence spread. On the contrary, the secretion of EVs by the host cells may be a method to expel intracellular bacteria, neutralize bacterial toxins, or stimulate both innate and adaptive immune responses.
- Immunoregulatory activity of EVs is related to their biological content, which consists of molecules known to be involved in the regulation of immune cells. As an example, heat shock proteins (HSP) that were shown to be present in EVs are known immunomodulators. Apart from the possible ways of EV-mediated activation of the immune system, several findings demonstrate their immunosuppressive role in homeostasis and disease.
RELATED PRODUCTS & SERVICES
Reference
- Karnas E, et al. (2023). "Stem cell-derived extracellular vesicles as new tools in regenerative medicine - Immunomodulatory role and future perspectives." Front Immunol. 14, 11201759.
For research use only. Not for any other purpose.




