Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
MC-38
Cat.No.: CSC-6983J
Species: C57BL6 mouse
Morphology: Epithelial
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
MC38 is a mouse colon adenocarcinoma cell line originally derived from a colon tumor induced by the chemical carcinogen azoxymethane in C57BL/6 mice. These cells have been widely used as a model system for studying various aspects of colon cancer biology, including the molecular mechanisms underlying tumor development and progression, and the identification of potential therapeutic targets for the treatment of colon cancer.
MC38 cells have several properties that make them useful for research. Firstly, they are relatively easy to culture in the laboratory and can be grown in large quantities. Secondly, they express markers of colon cancer, such as carcinoembryonic antigen (CEA) and cytokeratin, and exhibit several characteristics of colon cancer, including uncontrolled proliferation, invasion, and metastasis. Thirdly, they respond to various stimuli, such as cytokines and growth factors, making them a useful model system to study the effects of these factors on the growth and survival of colon cancer cells.
MC38 cells have been used to study the role of various signaling pathways, such as the Wnt/β-catenin pathway, in the development and progression of colon cancer. In addition, MC38 cells have been used to screen for potential anticancer agents, including chemotherapeutic drugs and targeted therapies. MC38 cells have been used to study the mechanisms underlying drug resistance in colon cancer. Understanding the mechanisms of drug resistance in MC38 cells has led to the development of combination therapies and targeted therapies that can overcome drug resistance in colon cancer. The MC38 syngeneic mouse model encompasses a full immune system that is not available in human cell line derived xenograft models. This is extremely beneficial for preclinical studies focusing on novel immunotherapies that target immune checkpoint inhibitors or activate the immune system.
Characterization of Snail-MC38 Stable Clones
Stable transfection of MC38 with the Snail-pcDNA3.1 vector was performed as described previously. Two positive clones were generated through G418 selection, i.e., Snail-overexpressing MC38 clones #2 and #6 (Snail 2, Snail 6), and compared throughout the study with control pcDNA-MC38 cells (Mock).
The levels of Snail expression were evaluated in pcDNA-MC38 (Mock) and Snail-overexpressing (Snail-MC38) clones #2 and #6 by real-time PCR and Western blot analyses (Fig. 1A, B). E-cadherin (E-CADH) and integrin β1 expression levels were decreased in Snail-MC38 clone #6. Increase of β-catenin (β-CTN) expression in that clone was also observed (Fig. 1C). Further, as presented in Fig. 1D, Snail-MC38 cells acquired a spindle and dendritic shape, and cell-to-cell contacts became loose. We also performed a proliferation assay comparing Snail-MC38 clones with Mock cells (Fig. 1D). No significant differences were detected.
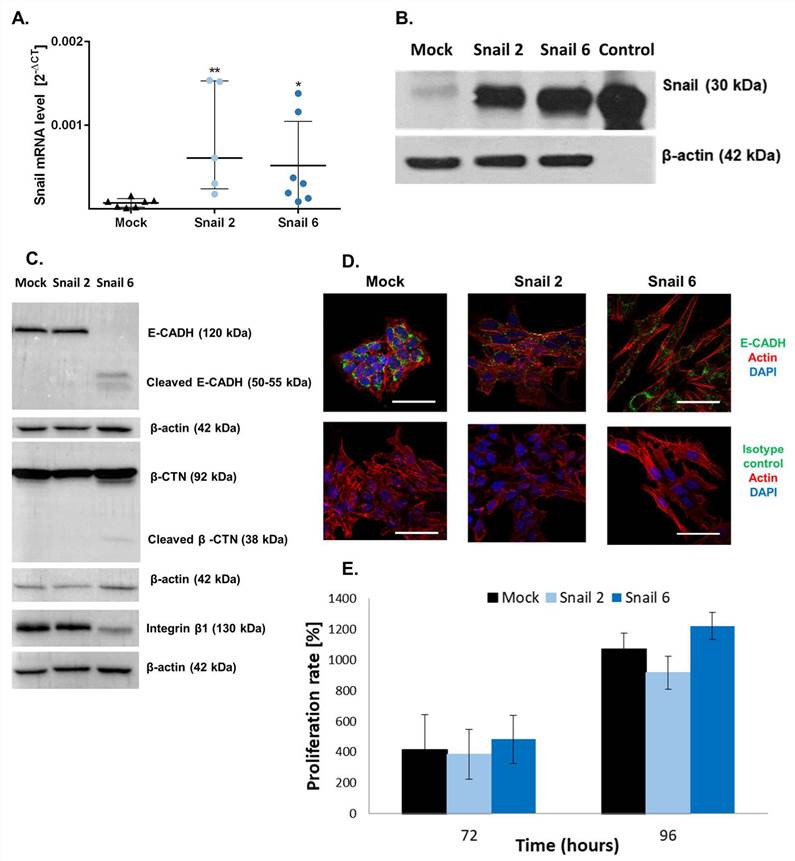 Fig. 1. Characterization of Snail-MC38 stable clones (Papiewska-Pająk, Izabela, et al., 2020).
Fig. 1. Characterization of Snail-MC38 stable clones (Papiewska-Pająk, Izabela, et al., 2020).
Effect of Snail Overexpression in MC38 Cells on MMPs Activities and Gene Expression of Proteoglycans
As presented in Fig. 2A, in mouse MC38 cells, Snail upregulated the level of gelatinase pro-MMP-9, while the level of MMP-2 was not affected, as evaluated by gelatin zymography. The level of MMP-14 protein (Fig. 2B) and its activity (Fig. 2C) was increased in both Snail-MC38 clones. Potentiation of MMP-14 activity by Snail was previously observed in B16F1 melanoma cells, and this effect was inhibited by Lumican (LUM). In contrast to Snail-B16F1 cells, the increased MMP-14 activity in Snail-MC38 cells was not affected by the presence of LUM. This result led us to investigate whether LUM was endogenously expressed in Mock- or Snail-MC38 cells. As shown in Fig. 2D, Lum gene expression was barely detectable by RT-PCR and equal in all MC38 clones. In both Mock- and Snail-MC38 clones, we also analyzed gene expression of other proteoglycans that are known to impact cancer progression. Gpc1 and syndecan-4 (Sdc4) were the two proteoglycans with the most elevated gene expression in MC38 cells, but the overexpression of Snail did not exert any effect (Fig. 2D).
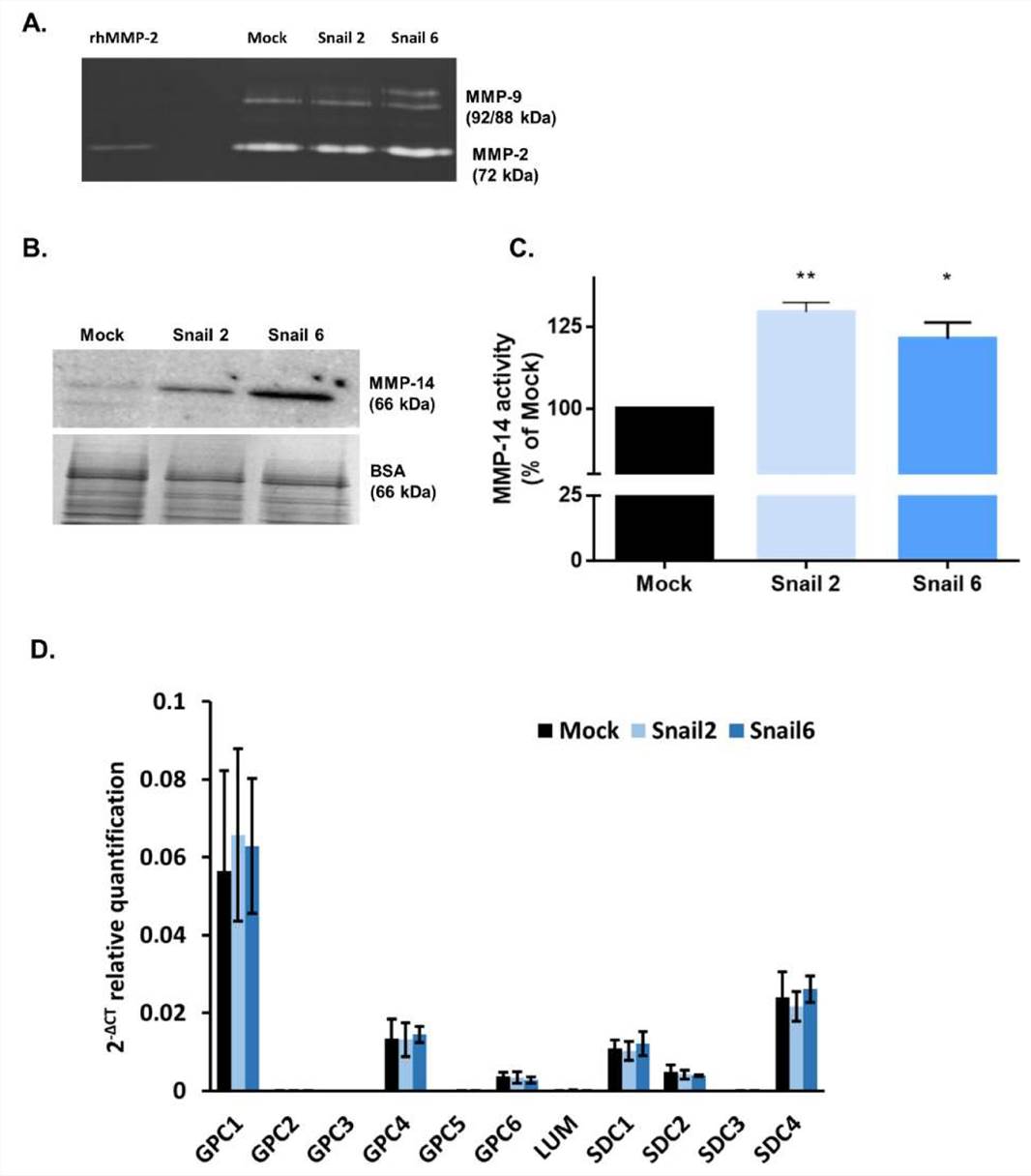 Fig. 2. Effect of Snail on matrix metalloproteinase (MMP)-2, -9, and -14 activity and proteoglycan gene expression in MC38 cells (Papiewska-Pająk, Izabela, et al., 2020).
Fig. 2. Effect of Snail on matrix metalloproteinase (MMP)-2, -9, and -14 activity and proteoglycan gene expression in MC38 cells (Papiewska-Pająk, Izabela, et al., 2020).
Comparison of immunogenic level between MC38-L and MC38-K colon carcinoma cell lines
Despite both MC38-L and MC38-K cell lines being of the same origin, MC38, and possessing some mutations in common, they can be distinguished based on the expression of cell-line specific mutations such as AdpgkR304M and Rpl18Q125R. The mutations in Adpgk and Rpl18 induced endogenous CD8+ T cell responses when MC38-L tumors regressed in mice treated with αPDL1 and splenocytes were expanded ex vivo upon recurrent stimulation with irradiated MC38-L cells (Fig. 3A) to generate antigen-specific CD8+ T cell lines. Coculture of established AdpgkR304M or Rpl18Q125R specific CD8+ T cell lines (Fig. 3A) with MC38-L and MC38-K cells showed a strongly reduced capacity of the T cells to recognize MC38-K cells (Fig. 3B).
To further explore this difference between MC38-L and MC38-K cells on the immunological level, we engineered T cells expressing TCRs against AdpgkR304M or Rpl18Q125R neoantigens and evaluated IFNγ secretion as well as cytotoxicity by TCR-specific T cells upon co-culture with tumor cells. Upon stimulation, Adpgk-TCR transduced T cells recognized MC38-L but not MC38-K cells (Fig. 3C). After co-culture with IFNγ pre-stimulated MC38-L cells, Rpl18-TCR transduced T cells also showed tumor recognition (Fig. 3D). IFNγ pre-stimulation of MC38-L cells prior to co-culture with TCR-transduced T cells resulted in an increase (>50%) in the number of IFNγ spots (Fig. 3C, D). The number of IFNγ spots was comparable between MC38-K, with or without IFNγ pre-stimulation, and B16-Ova cells, our control cell line, pointing out that MC38-K cell line is not recognized by T cells of AdpgkR304M or Rpl18Q125R neoantigens specificity. Only forced expression of these neoantigens but not OvaI257-264 in MC38-K cells via electroporation of matching neoantigen encoding RNAs resulted in significant recognition of the tumor cells by Adpgk- or Rpl18-TCR transduced T cells (Fig. 3C, D).
Following tumor cell recognition via IFNγ ELISPOT, we also tested in vitro cytotoxic effects of TCR-transduced T cells on tumor cells. Adpgk- and Rpl18-TCR transduced T cells resulted in 40% and 20% lysis of MC38-L cells, respectively (Fig. 3E). TCR-transduced T cells caused lysis of MC38-K cells only when these cells were forced to express the matching antigens for the TCRs. Otherwise, the percentage of lysed cells by neoantigen-specific TCRs was similar between MC38-K and B16-Ova cells.
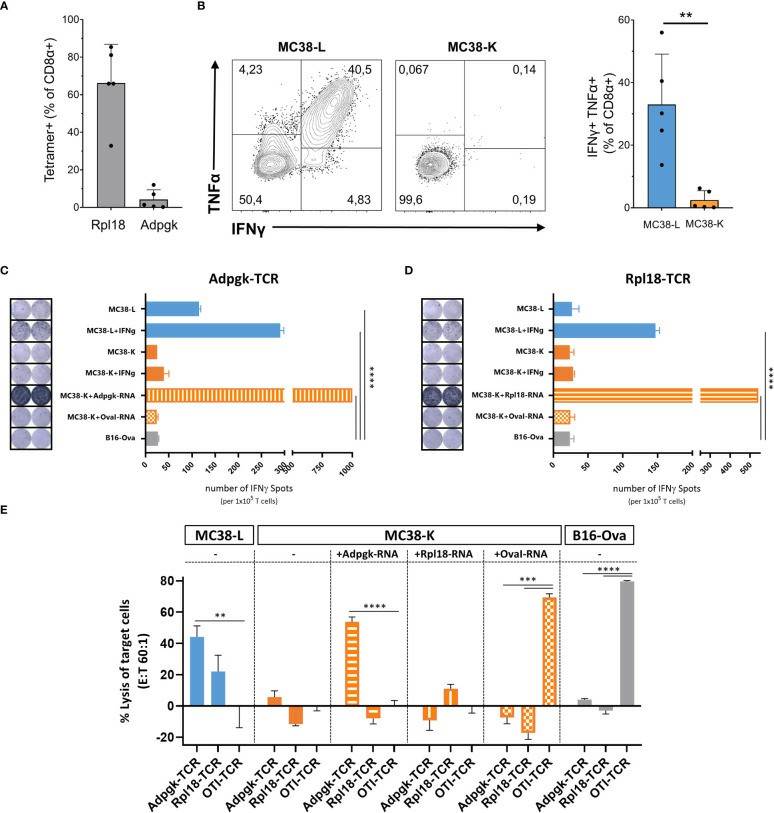 Fig. 3. Comparison of MC38-L and MC38-K cell lines on immunogenic level (Schrörs, Barbara, et al., 2023).
Fig. 3. Comparison of MC38-L and MC38-K cell lines on immunogenic level (Schrörs, Barbara, et al., 2023).
Ask a Question
Write your own review