Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
Human iPSC-derived Schwann Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Schwann cells function as the main glial cell type within the peripheral nervous system where they myelinate axons and repair nerves. Schwann cell malfunction leads to neurodegenerative conditions like Charcot-Marie-Tooth disease, diabetic peripheral neuropathy, and Guillain-Barré syndrome. The lack of effective in vitro models poses challenges in studying these diseases' mechanisms and developing treatments. hiPSC technology creates new opportunities to resolve this problem by generating Schwann cells from hiPSCs through directed differentiation. The hiPSC differentiation process involves sequential steps starting from neural crest-like cell generation, moving to Schwann cell precursor formation and finally achieving mature Schwann-like cell development. hiPSC-derived Schwann cells share both structural and functional characteristics with human primary Schwann cells. The cells show expression of Schwann cell markers including SOX10, P75NTR, GAP43, S100B and MBP. These cells can carry out myelination which leads to the formation of myelin protein deposits including MBP and MOG.
hiPSC-derived Schwann cells demonstrate versatile potential for applications across diverse scientific areas. Scientists can study neurodegenerative disease mechanisms by simulating the differentiation of Schwann cells within specific genetic frameworks. For instance, Schwann cell generation from hiPSCs of CMT1A patients facilitates the investigation of molecular disease mechanisms. These cells also serve as research tools for nerve injury repair studies and myelin regeneration experiments which lead to novel clinical therapeutic approaches.
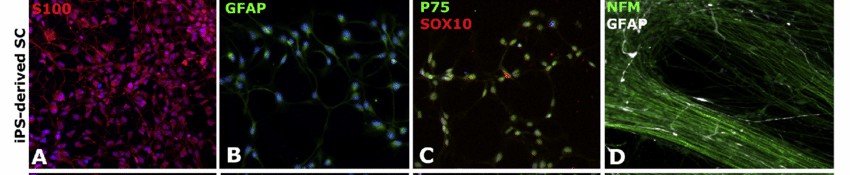 Fig. 1. Human iPSC-derived Schwann cells (A-D) were stained in 2D with the specific markers S100 (in red; A), GFAP (in green; B), P75 (in green; C) and SOX10 (in red; C). (in white with GFAP and green with NFM for 18 days; D) (Muller Q, Beaudet M, et al., 2018).
Fig. 1. Human iPSC-derived Schwann cells (A-D) were stained in 2D with the specific markers S100 (in red; A), GFAP (in green; B), P75 (in green; C) and SOX10 (in red; C). (in white with GFAP and green with NFM for 18 days; D) (Muller Q, Beaudet M, et al., 2018).
Functional Equivalence of hESC- and iPSC-derived Schwann Cells
Traditional methods of obtaining Schwann cells from human biopsies are invasive and yield limited cells. Stem cell differentiation, especially from pluripotent stem cells, provides an efficient alternative. Though human embryonic stem cells (hESCs) are considered ideal due to their pluripotent nature, ethical concerns pivot the focus towards human induced pluripotent stem cells (hiPSCs). However, the equivalence of hESCs and hiPSCs remains debated.
To assess the functional equivalence of hESC- and hiPSC-derived Schwann cells, a chronic denervation and regeneration mouse model was utilized. Both alive and heat killed Schwann cells were transplanted. Histological, electrophysiological, and behavioral analyses evaluated nerve regeneration. The number of myelinated axons regenerated at the repair site was similar between alive hESC- and hiPSC-derived Schwann cells (Fig. 1A and B). Alive cells significantly improved regeneration compared to heat-killed cells. CMAPs from foot muscles showed comparable results for amplitude and latency with alive cells (Fig. 1C-E) and better outcomes than heat-killed cells. Skilled walking tests further confirmed similar benefits in nerve and muscle function. Consequently, transplantation tests used one hESC and one hiPSC line, H9 and GM01582, respectively. The results suggest both Schwann cell types equally enhance axon regeneration and muscle reinnervation.
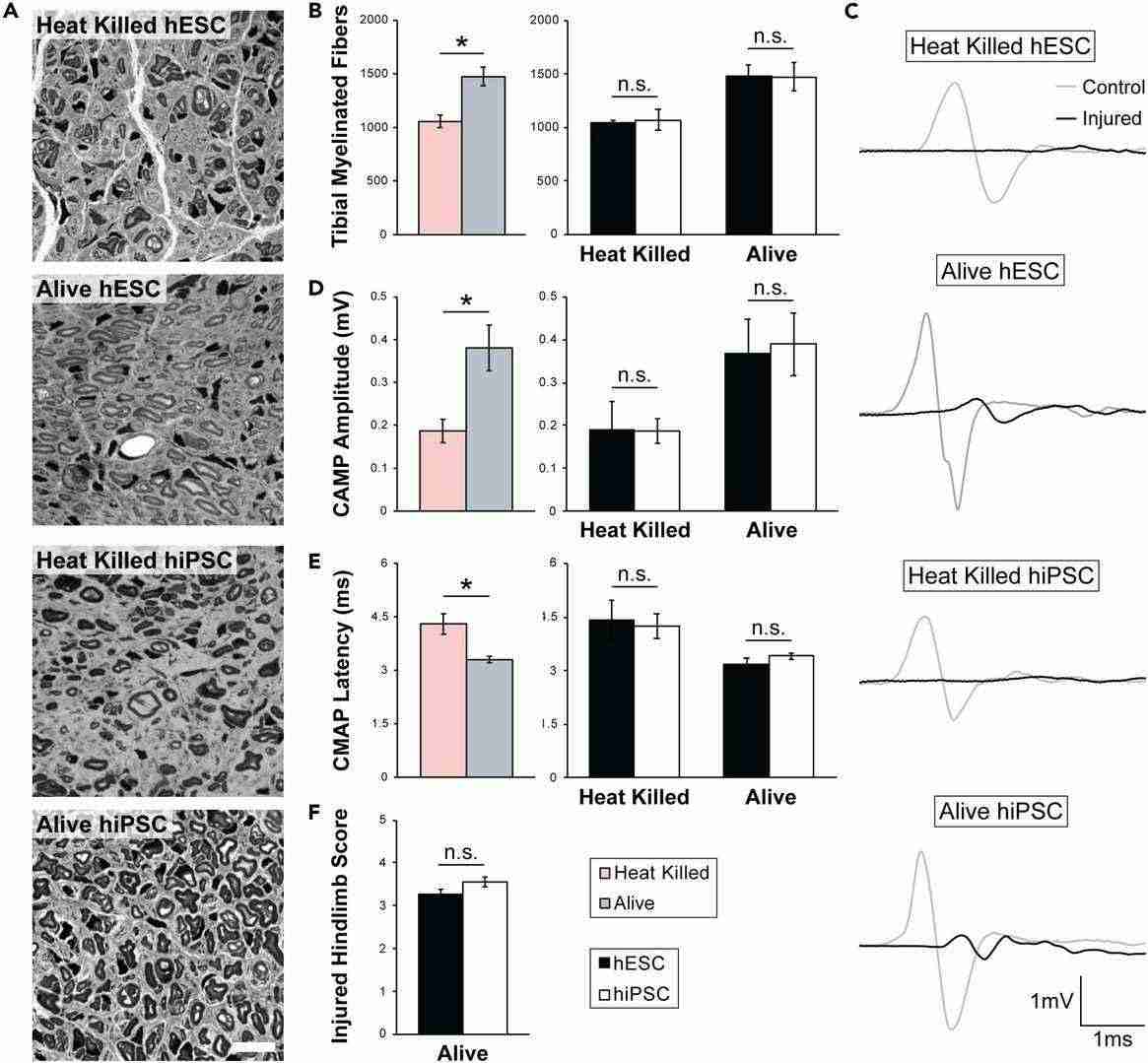 Fig. 1. hESC- and hiPSC-derived Schwann cells equally promote regeneration of chronically denervated peripheral nerves (Moss K R, Mi R, et al., 2024).
Fig. 1. hESC- and hiPSC-derived Schwann cells equally promote regeneration of chronically denervated peripheral nerves (Moss K R, Mi R, et al., 2024).
Higher Cell Proliferation Level and Abnormal Cell Morphology in NF2L64P-Derived SCs
NF2-Related Schwannomatosis is a genetic disease caused by mutations in the NF2 gene, leading to the loss of the tumor suppressor protein, merlin. This results in the formation of schwannomas, benign tumors affecting the peripheral nervous system and impacting hearing and balance. Tang et al. employ a human iPSC-derived Schwann cell model to investigate the role of the merlin protein in human Schwann cells, aiming to uncover key molecular interactions.
They established a hiPSC line with the homozygous NF2 mutation, p.L64P, and differentiated Schwann cells (SCs) from both NF2L64P and isogenic wild-type NF2WT lines using a modified protocol (Fig. 2A). By day 18, Schwann cell progenitors (SCPs) from both lines showed similar SOX10 expression and morphology (Fig. 2B). At maturation day 14, approximately 80% of cells in both lines were S100β+ SCs (Fig. 2C), with 5-10% being neurons (Fig. 2D). Interestingly, NF2L64P hiPSC-derived Schwann cells (SCs) showed significantly higher proliferation compared to NF2WT SCs, as indicated by BrdU assays on maturation day 14 (Fig. 3A). Morphologically, NF2WT SCs displayed a bipolar shape with small ruffles at the poles (Fig. 3B), while NF2L64P SCs lacked this polarity, exhibiting a more spread-out shape with larger ruffles and greater cytoplasmic volume (Fig. 3B). The cell area measurements confirmed larger sizes in NF2L64P SCs (Fig. 3C). These results indicate that the NF2 mutation p.L64P in hiPSC-derived SCs leads to increased proliferation and distinct morphological changes, consistent with previous studies.
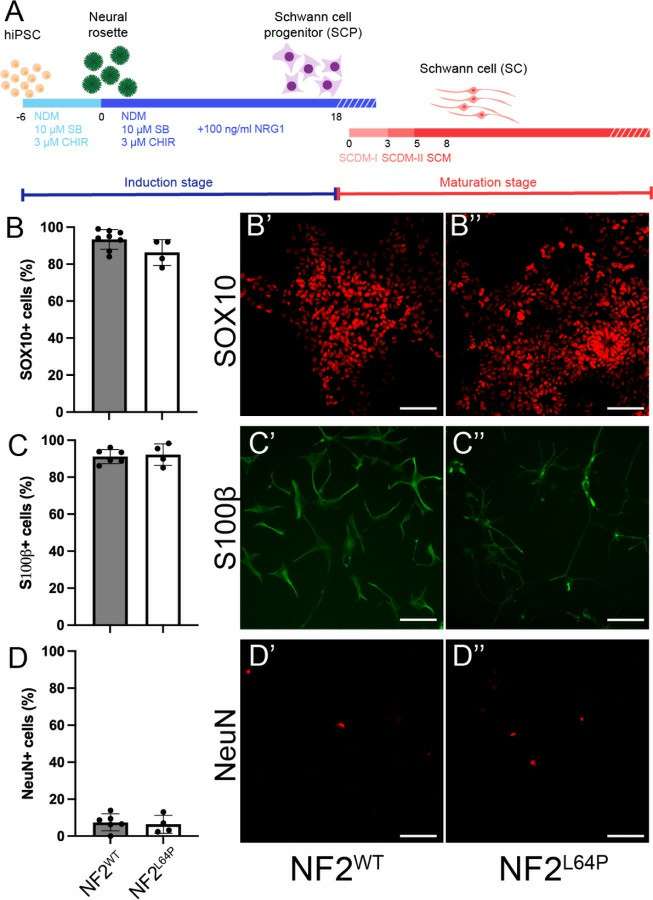 Fig. 2. Differentiation of Schwann cells (SCs) from NF2WT and NF2L64P hiPSC lines (Tang PC, Um S, et al., 2024).
Fig. 2. Differentiation of Schwann cells (SCs) from NF2WT and NF2L64P hiPSC lines (Tang PC, Um S, et al., 2024).
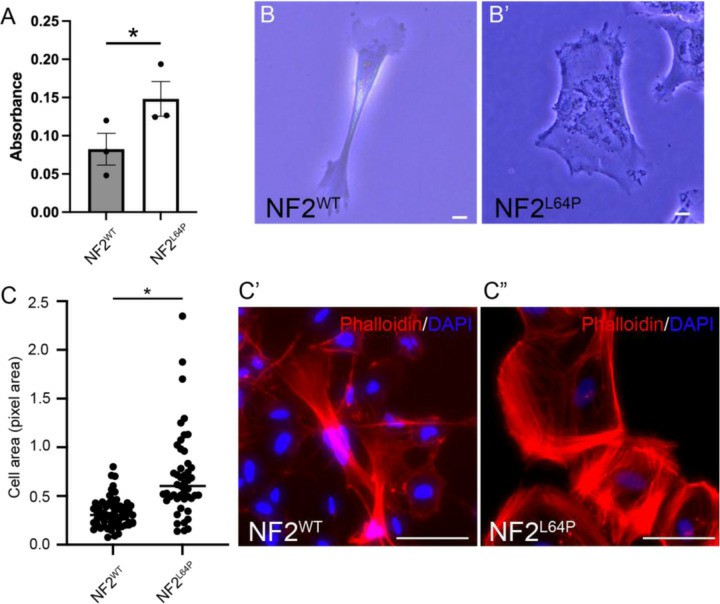 Fig. 3. Abnormal phenotypes in NF2L64P-derived Schwann cells (SCs) (Tang PC, Um S, et al., 2024).
Fig. 3. Abnormal phenotypes in NF2L64P-derived Schwann cells (SCs) (Tang PC, Um S, et al., 2024).
Ask a Question
Write your own review

