- You are here: Home
- Disease Models
- Skin Disease Models
- Hair Growth Models
- Alopecia Areata (AA) Models
- Cyclophosphamid-Induced Alopecia Areata (AA) Model
Disease Models
- Oncology Models
-
Inflammation & Autoimmune Disease Models
- Rheumatoid Arthritis Models
- Glomerulonephritis Models
- Multiple Sclerosis (MS) Models
- Ocular Inflammation Models
- Sjögren's Syndrome Model
- LPS-induced Acute Lung Injury Model
- Peritonitis Models
- Passive Cutaneous Anaphylaxis Model
- Delayed-Type Hypersensitivity (DTH) Models
- Inflammatory Bowel Disease Models
- Systemic Lupus Erythematosus Animal Models
- Asthma Model
- Sepsis Model
- Psoriasis Model
- Atopic Dermatitis (AD) Model
- Scleroderma Model
- Gouty Arthritis Model
- Carrageenan-Induced Air Pouch Synovitis Model
- Carrageenan-Induced Paw Edema Model
- Experimental Autoimmune Myasthenia Gravis (EAMG) Model
-
Cardiovascular Disease Models
- Surgical Models
- Animal Models of Hypertension
- Venous Thrombosis Model
- Atherosclerosis model
- Cardiac Arrhythmia Model
- Hyperlipoidemia Model
- Doxorubicin-induced Heart Failure Model
- Isoproterenol-induced Heart Failure Model
- Arterial Thrombosis Model
- Pulmonary Arterial Hypertension (PAH) Models
- Heart Failure with Preserved Ejection Fraction (HFpEF) Model
-
Neurological Disease Models
- Alzheimer's Disease Modeling and Assays
- Seizure Models
- Parkinson's Disease Models
- Ischemic Stroke Models
- Acute Spinal Cord Injury (ASCI) Model
- Traumatic Brain Injury (TBI) Model
- Hypoxic-Ischemic Encephalopathy (HIE) Model
- Tourette Syndrome (TS) Model
- Amyotrophic Lateral Sclerosis (ALS) Model
- Huntington's Disease (HD) Model
- Intracerebral hemorrhage (ICH) Models
- Pain Models
- Metabolic Disease Models
- Liver Disease Models
- Rare Disease Models
- Respiratory Disease Models
- Digestive Disease Models
-
Urology Disease Models
- Cisplatin-induced Nephrotoxicity Model
- Unilateral Ureteral Obstruction Model
- 5/6 Nephrectomy Model
- Renal Ischemia-Reperfusion Injury (RIRI) Model
- Diabetic Nephropathy (DN) Models
- Passive Heymann Nephritis (PHN) Model
- Adenine-Induced Chronic Kidney Disease (CKD) Model
- Kidney Stone Model
- Doxorubicin-Induced Nephropathy Model
- Orthopedic Disease Models
- Ocular Disease Models
- Skin Disease Models
- Infectious Disease Models
Cyclophosphamid-Induced Alopecia Areata (AA) Model
Cyclophosphamide, a commonly used chemotherapeutic agent, is known to induce hair loss as a side effect due to its effects on rapidly dividing cells. While it effectively targets cancer cells, it also inadvertently impacts the rapidly dividing cells surrounding hair follicles, making it a useful tool for preparing alopecia areata models.
Creative Bioarray has developed an innovative animal model of alopecia areata induced by cyclophosphamide, which allows researchers to study the pathogenesis of this condition and evaluate potential treatments. This model is characterized by its ability to sustain the induced hair loss for approximately 20 days, providing a stable and reproducible platform for in-depth research into alopecia areata mechanisms and therapeutic interventions.
Our Cyclophosphamid-Induced Alopecia Areata (AA) Model
Available Animal
Mouse
Modeling Method
Except for the mice in control group which are given an equal volume of normal saline, the mice in model group are intraperitoneally injected with a single dose of freshly dissolved cyclophosphamide saline on the 9th day after hair removal.
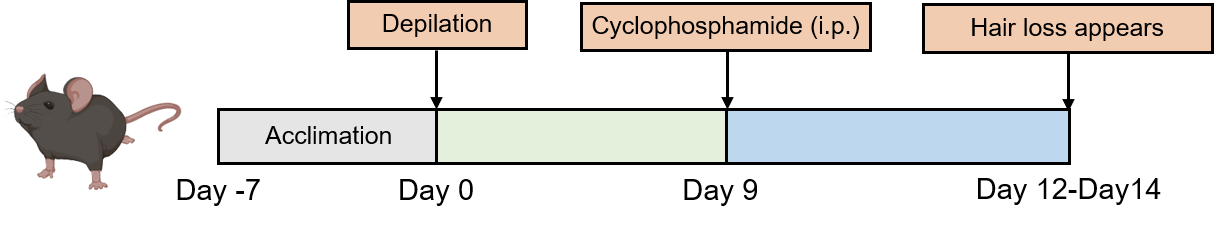 Fig. 1 Modeling method of cyclophosphamid-induced alopecia areata (AA) in Creative Bioarray
Fig. 1 Modeling method of cyclophosphamid-induced alopecia areata (AA) in Creative Bioarray
Endpoints
- Image of mice
- Hair loss area
- Histology analysis
- qPCR or Western blot
- Customized endpoints tailored to your specific research requirements
Example Data
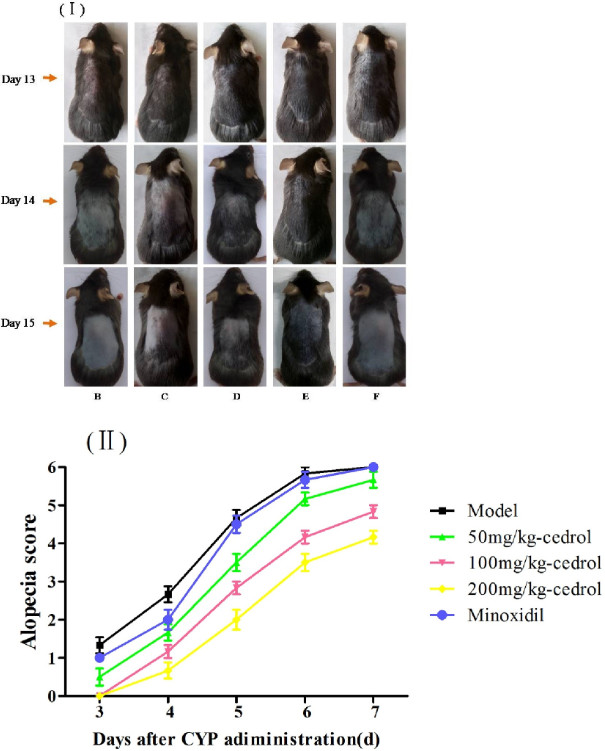 Fig.2 (I): Macroscopic effects among the groups. (B) Model group; (C) 50 mg/kg-cedrol group; (D) 100 mg/kg-cedrol group; (E) 200 mg/kg-cedrol group; (F) Minoxidil group (Photos were taken on day 13, 14 and 15 of the experiment respectively). (II): Alopecia score of the mice after CYP administration. (Chen et al. 2016)
Fig.2 (I): Macroscopic effects among the groups. (B) Model group; (C) 50 mg/kg-cedrol group; (D) 100 mg/kg-cedrol group; (E) 200 mg/kg-cedrol group; (F) Minoxidil group (Photos were taken on day 13, 14 and 15 of the experiment respectively). (II): Alopecia score of the mice after CYP administration. (Chen et al. 2016)
Quotation and Ordering
Equipped with extensive experience in researching animal models of diseases, the experts at Creative Bioarray are willing to collaborate with you in crafting a customized design tailored to your precise needs. If you are interested in our services, please feel free to contact us at any time or submit an inquiry to us directly.
Reference
- Chen, S.S., et al. Preventive effects of cedrol against alopecia in cyclophosphamide-treated mice. Environmental Toxicology and Pharmacology, 2016, 46: 270-276.
For research use only. Not for any other purpose.

