Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
GF-D8
Cat.No.: CSC-C0614
Species: Human
Source: acute myelocytic leukemia
Morphology: round to polymorph cells growing in suspension
Culture Properties: suspension
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Immunology: CD3 -, CD13 +, CD14 -, CD15 -, CD19 -, CD33 +, CD34 -, HLA-DR -
Viruses: PCR: EBV -, HBV -, HCV -, HIV -, HTLV-I/II -, SMRV -
The GF-D8 cell line is a valuable model for the study of a subtype of acute myelocytic leukemia (AML). This cell line was established from the peripheral blood of an 82-year-old male patient who was diagnosed with AML M1, a specific morphological and immunophenotypic classification of AML, in 1989.
One of the defining features of the GF-D8 cell line is its growth dependence on granulocyte-macrophage colony-stimulating factor (GM-CSF) or interleukin-3 (IL-3). These cytokines play crucial roles in the regulation of myeloid cell development and differentiation. The GF-D8 cells require the presence of either GM-CSF or IL-3 in the culture medium to proliferate, which reflects the growth factor dependency often observed in AML cells.
By analyzing the response of GF-D8 cells to various stimuli and therapeutic interventions, scientists can gain valuable insights into the disease biology and identify potential targets for novel treatment strategies. Furthermore, the GF-D8 cell line serves as a useful tool for testing the efficacy and mechanisms of action of novel anti-leukemic agents, particularly those targeting the growth factor signaling pathways that are essential for the survival and proliferation of these malignant cells.
Establishment and Characterization GF-D8 Cell Lines
A novel factor-dependent human myeloid leukemia cell line (GF-D8) was established from the peripheral blood of an 82-year-old man suffering from AML. The morphologic appearance of the GF-D8 cell line is consistently that of immature poorly differentiated myeloid blasts. The cells are usually round with a basophilic cytoplasm, full of azurophilic granules with few vacuoles and no Auer rods; the nucleus is large, round, or slightly irregular (Fig. 1A). Electron microscopy shows most of the cells have a single, large, central, or eccentric nucleus with a single nucleolus and a low to moderate chromatin condensation. In many cells, the nucleus is irregularly convoluted or lobed. The great majority of cells contain numerous developed primary granules in the cytoplasm, associated with vacuoles filled with low-density material, predominant in some cells. Further, dense haloed structures are intermingled with the primary granules in a significant number of cells (Fig. 1B). The consensus karyotype found in GF-D8 cells is 45 XY, -5, del(7) (q32qter), inv(7) (q31.2q36). 8q+, +8q+, 11q+, 12p-, -15, -17+ marker chromosome.
To demonstrate the lack of lymphoid commitment, the configuration of TCR-δ and IgH loci was studied. As shown in Fig. 2A. GF-D8 had conserved either for TCR-δ and IgH both alleles in the germline configuration. For the karyotypic abnormalities involving chromosome 8. the presence of c-myc amplification was investigated by Southern blot (Fig. 2C). After normalization with the actin gene, scanning of the autoradiograms showed that the amount of c-myc DNA in GF-D8 cells was 4.3-fold more abundant than in control cells. Southern blot hybridization showed no bands specific to EBV (Fig. 2D).
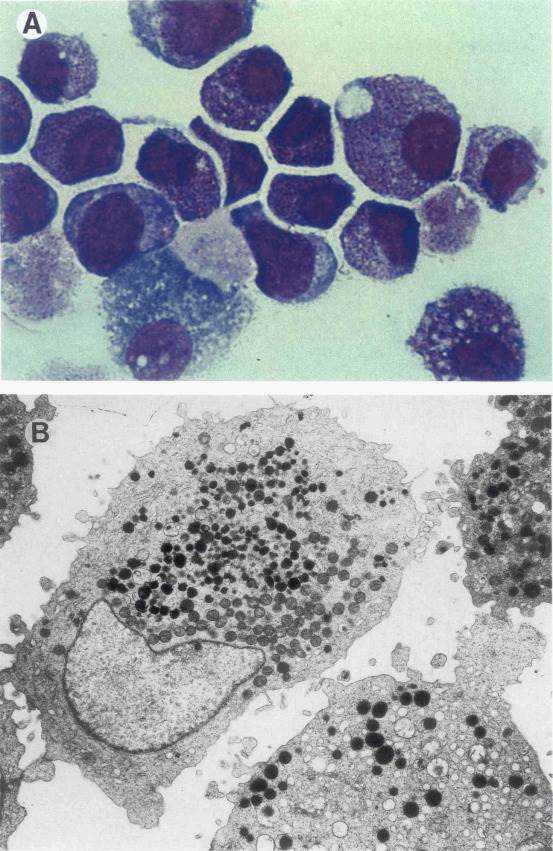 Fig. 1 Cell morphology of the GF-D8 cell line. (Rambaldi A, et al.,1993)
Fig. 1 Cell morphology of the GF-D8 cell line. (Rambaldi A, et al.,1993)
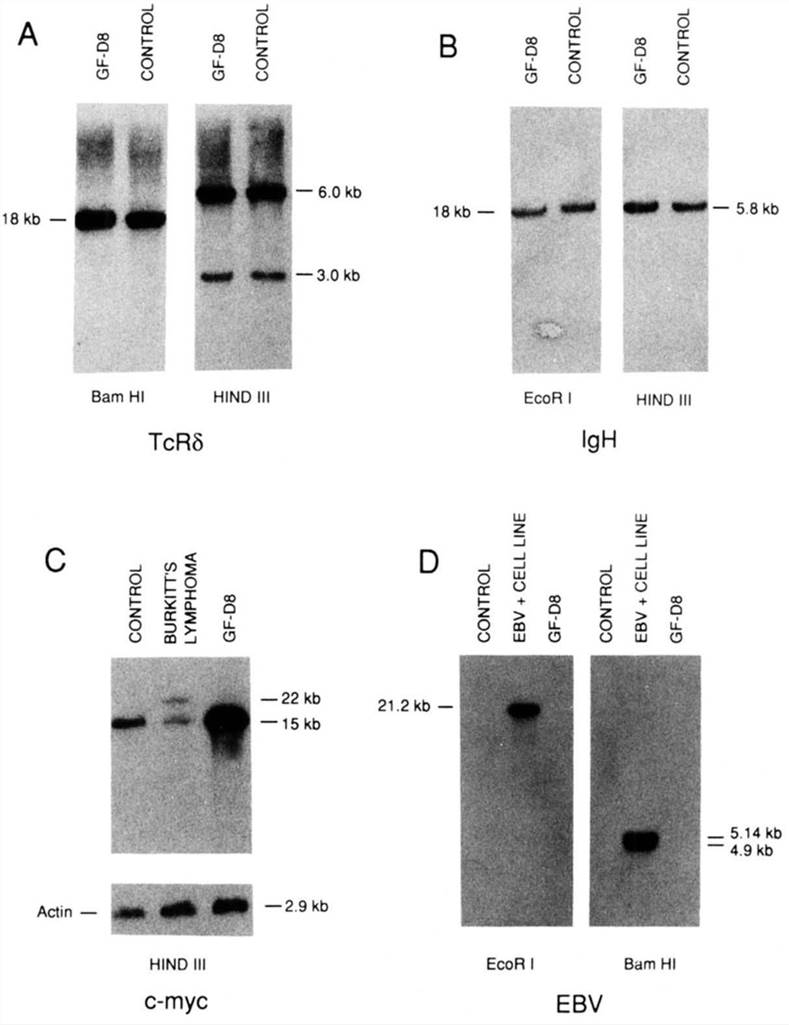 Fig. 2 Southern blot analysis of GF-D8 cells. (Rambaldi A, et al.,1993)
Fig. 2 Southern blot analysis of GF-D8 cells. (Rambaldi A, et al.,1993)
Expression of c-myb and B-myb genes in GF-D8 Cells
Direct and indirect evidence strongly indicates that the proto-oncogene c-myb plays an important role in the regulation of both the proliferation and differentiation of hematopoietic cells. In addition, recent data suggest that the structurally related B-myb gene is also necessary for the proliferation of these cells.
Total RNA was isolated from resting (cultured in 1% FCS without GM-CSF) and proliferating cells (grown in 10% FCS and GM-CSF) and analyzed in Northern blots (Fig. 3E and F). As shown in Fig. 3C and E, B-myb mRNA gradually decreased after the removal of GM-CSF and high serum concentration, becoming virtually undetectable at day 4. B-myb mRNA. on the other hand, rather increased in the cells seeded in the presence of GM-CSF and high serum, presumably reflecting the proliferation of these cells induced by the added growth factors (Fig. 3A). However, c-myb RNA levels remained approximately constant either in the presence or in absence of GM-CSF and serum (Fig. 3C and E). They were similar to the levels of the β-actin control (Fig. 3E).
After 4 days of starvation, the GM-CSF-deprived cells were induced to proliferate by adding to the culture medium GM-CSF and 10% FCS. This stimulated them to reenter the cell cycle and proliferate. Such stimulation was accompanied by a strong simultaneous induction of thymidine uptake (Fig. 3B) and of B-myb RNA (Fig. 3D and F), whereas c-myb RNA levels remained constantly high under all these conditions (Fig. 3D and F). Similar results were obtained when analyzing the p75c-myb and p93B-myb proteins under the same experimental conditions (Fig. 3G). B-myb protein had become already undetectable 2 days after the removal of GM-CSF and was reinduced 1 day after the addition of the growth factor to the starved cells, whereas c-myb protein levels remained constant throughout the experiment (Fig. 3G).
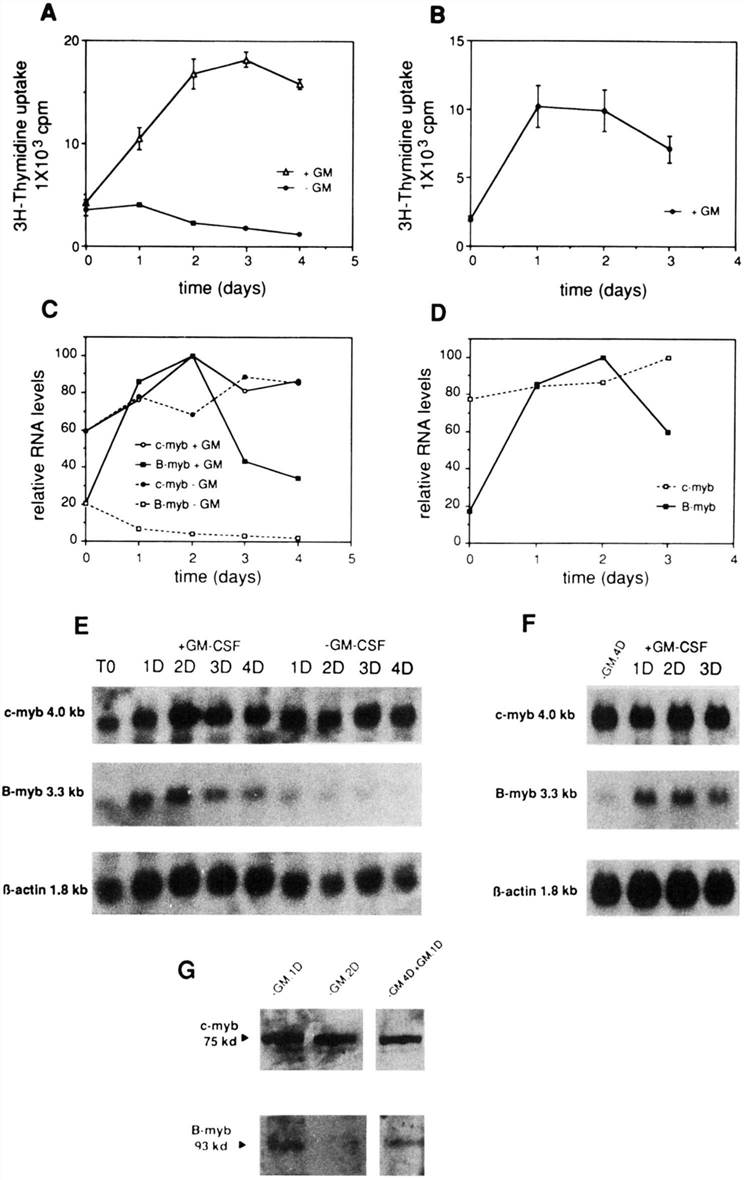 Fig. 3 Patterns of c-myb and B-myb expression in proliferating and growth-arrested GF-D8 cell line. (Arsura M, et al., 1994)
Fig. 3 Patterns of c-myb and B-myb expression in proliferating and growth-arrested GF-D8 cell line. (Arsura M, et al., 1994)
Karyotyping, traditionally performed using cytogenetic techniques, is indispensable for validating genome assemblies whose sequence lengths can be scaled up to chromosome sizes using modern methods.
The GF-D8 cell line is growth-dependent on the presence of either GM-CSF or IL-3 in the culture medium.
The growth factor dependency of GF-D8 cells reflects the often observed aberrant signaling pathways in AML cells, which can lead to their uncontrolled proliferation and survival.
The GF-D8 cell line is a valuable tool for investigating the underlying molecular mechanisms of AML M1, as well as for testing the efficacy and mechanisms of action of novel anti-leukemic agents.
Ask a Question
Average Rating: 5.0 | 3 Scientist has reviewed this product
Easy ordering
The ease of ordering and timely delivery of Creative Bioarray has expedited my experimental process.
15 Feb 2023
Ease of use
After sales services
Value for money
Reliable products
I am confident in the integrity of my research data thanks to the reliable GF-D8 cells I have obtained from this provider, and I plan to continue using their services for future projects.
24 Feb 2024
Ease of use
After sales services
Value for money
Expected growth
The cells have consistently exhibited the expected growth kinetics and cytokine-dependent proliferation, which is essential for the validity of my findings.
23 Nov 2023
Ease of use
After sales services
Value for money
Write your own review
- You May Also Need