Immortalizing Cells for Cell-Cultured Meat
Cultured meat, also known as cultivated or lab-grown meat, represents a revolutionary approach to meat production in bioreactors that promises to address various environmental, ethical, and public health concerns associated with traditional animal agriculture. As the worldwide population and individual meat intake expands, the annual average global demand for meat is anticipated to rise by 1.3% from 2005 to 2050. The development of cultured meat products will require overcoming major technological challenges in adapting and developing bioengineering technology for food production, including bioreactors, cell culture media, and cell lines, as well as major challenges in consumer acceptance and new food regulations.
By partnering with companies who are looking to commercialize cultured meat, Creative Bioarray aims to offer our knowledge and manufacturing expertise to help propel them to overcome critical technological challenges. For cultured meat to succeed at scale, muscle cells must be expanded in vitro in a rapid and reliable manner. Toward this goal, immortalized cells offer substantial benefits over primary cells, including rapid growth, escape from cellular senescence, and consistent starting cell populations for production. We offer tailored Immortalized cell lines as valuable tools to the field, enabling further research and development to advance culture meat.
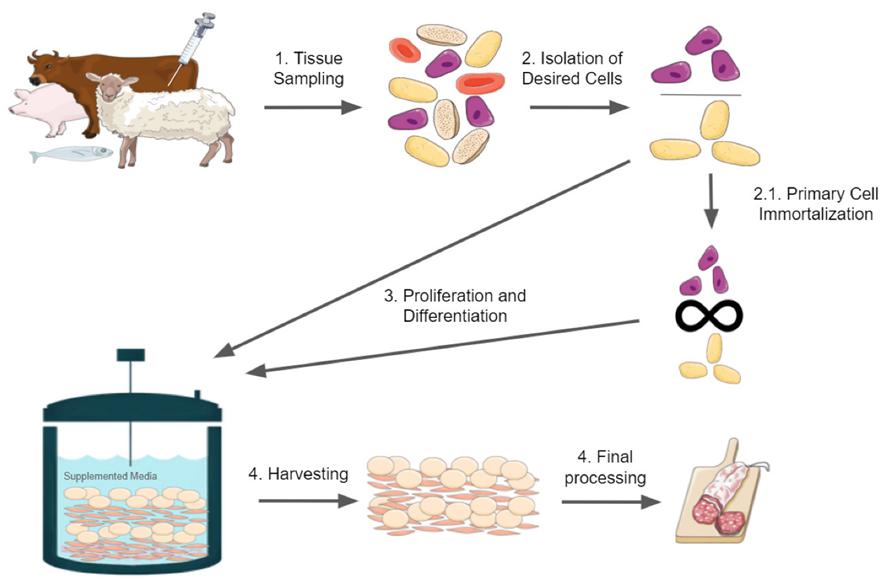 Figure 1. Brief overview of cultured meat production. The figure was constructed with illustrations taken from Servier Medical Art, licensed under a Creative Commons Attribution 3.0 Unported License.
Figure 1. Brief overview of cultured meat production. The figure was constructed with illustrations taken from Servier Medical Art, licensed under a Creative Commons Attribution 3.0 Unported License.
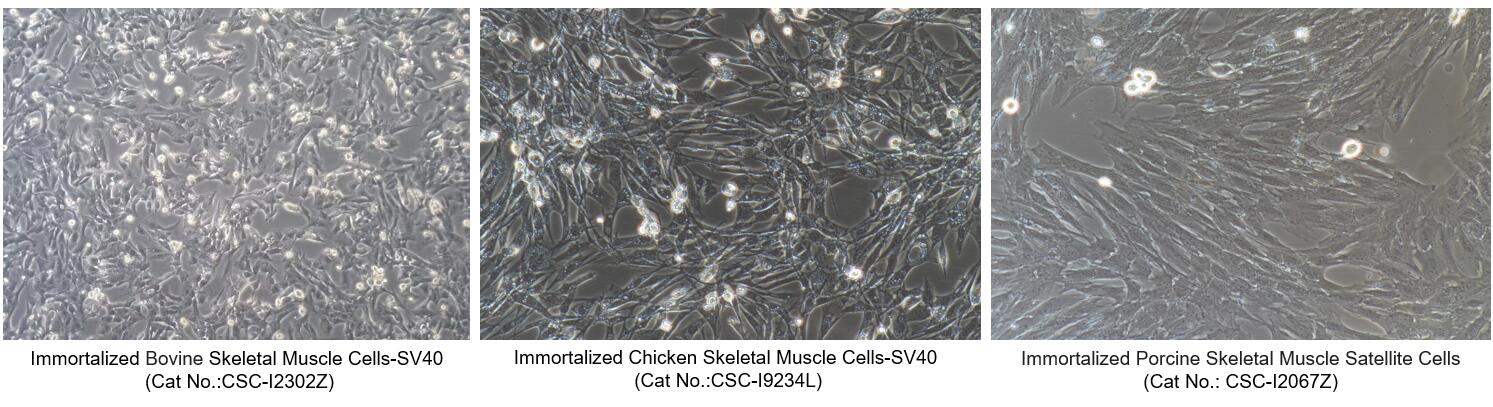 Figure 2. Morphology of Immortalized Animal Skeletal Muscle Cells from Creative Bioarray
Figure 2. Morphology of Immortalized Animal Skeletal Muscle Cells from Creative Bioarray
Immortalized Animal Skeletal Muscle Cells (In Stock):
| Cat No. | Product Name |
| CSC-I2302Z | Immortalized Bovine Skeletal Muscle Cells-SV40 |
| CSC-I9234L | Immortalized Chicken Skeletal Muscle Cells-SV40 |
| CSC-I2067Z | Immortalized Porcine Skeletal Muscle Satellite Cells-SV40 |
Advantages of Immortalized Cell Lines in Cell-Cultured Meat
For cultivated meat to eventually be widely accessible to consumers, the industry needs a constant supply of animal cells. So, primary cells can only undergo a finite number of cell divisions are not the most practical cells to use.
Stem cells are naturally immortal, an attractive characteristic for cultivated meat companies looking for a constant supply of cells. However, stem cells are expensive to culture and are extremely unstable.
Unlike primary and stem cell cultures, immortal cell lines do not undergo senescence, and can exhibit infinite divisions. They could, therefore, be easier to study, and allow for safer and more consistent cultured meat without ongoing need for animal biopsies.
Methods for Establishing Immortal Cell Lines
- Spontaneous Immortalization of Cell Lines
In rare events, most often in cancer, cells will spontaneously immortalize. These cells can then be isolated. However, this method has its limitation. Spontaneously immortalized cells may be held with concern as equivalent to cancerous cells.
- Overexpression of TERT Gene
The introduction of telomerase has been successful in immortalizing cell lines, because telomere elongation helps cells escape senescence triggered by telomere shortening. This has been performed by ectopic expression of the catalytically active subunit of telomerase (TERT), or by the overexpression of TERT. And some scientists developed genetically immortalized bovine satellite cells (iBSCs) via constitutive expression of bovine Telomerarse reverse transcriptase (TERT) and Cyclin-dependent kinase 4 (CDK4). These cells achieve over 120 doublings at the time of publication and maintain their capacity for myogeneic differentiation.
- Inactivation of the p53/p16/Rb Stress Response
The earliest method for immortalizing cell lines via inactivation or bypass of the p53/p16/Rb stress response has been transformation with viral genes. A notable example is the simian virus 40 (SV40) large T-antigen (TAg), which binds to and inactivates p53/Rb as well as other tumor suppressor factors in a number of species and organ types.
- Epigenetic Induction of Cell Growth
Many researchers worry about the genomic alteration due to viral-induced expression of SV40-T Antigen or other genes. The Epigenetic Induction of Cells Growth (No viral gene) is a great method to establish the immortalized cell lines.
Creative Bioarray's Immortalizing Cells for Cell-Cultured Meat Service has the following Features:
- Immortalized cell lines from any species and any tissues with the function you need
- Different methods of cell immortalization you can choose
- Characterization according to your requirements
- Projects are designed and handled by experienced scientists.
- Short turn-around time
- Competitive pricing
Quotation and ordering
Our customer service representatives are available 24hr a day! We thank you for considering Creative Bioarray as your Immortalizing Cells for Cell-Cultured Meat partner.
References
- Post, M.J.; Levenberg, S.; Kaplan, D.L.; Genovese, N.; Fu, J.; Bryant, C.; Negowetti, N.; Verzijden, K.; Moutsatsou, P. Scientific, sustainability and regulatory challenges of cultured meat. Nat. Food 2020, 1, 403–415.
- Hallman, W.K.; Hallman, W.K., II. An empirical assessment of common or usual names to label cell-based seafood products. J. Food Sci. 2020, 85, 2267–2277
- Labeling of Meat or Poultry Products Comprised of or Containing Cultured Animal Cells; Federal Register: Washington, DC, USA, 2021.
- Boler, D.; Woerner, D. What is meat? A perspective from the American Meat Science Association. Anim. Front. 2017, 7, 8–11.
- Alexandratos, N.; Bruinsma, J. World Agriculture Towards 2030/2050: The 2012 Revision; ESA Working Paper No. 12-03; FAO: Rome, Italy, 2012
- DiMaio, T.; This Scientist Is Developing New Cell Lines for Slaughter-Free Meat. The Good Food Institute. 2019.
- Maqsood, M.I.; Matin, M.M.; Bahrami, A.R.; Ghasroldasht, M.M. Immortality of Cell Lines: Challenges and Advantages of Establishment. Cell Biol. Int. 2013, 37, 1038–1045.
- Ben-Arye, T.; Levenberg, S. Tissue Engineering for Clean Meat Production. Front. Sustain. Food Syst. 2019, 3, 46.
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How Muscle Structure and Composition Influence Meat and Flesh Quality. Sci. World J. 2020, 2016, 3182746.
Explore Other Options

