ONLINE INQUIRY

Human Dermal Fibroblasts-adult (HDF-a)
Cat.No.: CSC-7798W
Species: Human
Source: Dermis; Skin
Morphology: Fibroblasts
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
HDF-a are isolated from adult human skin. HDF-a are cryopreserved at primary culture and delivered frozen. Each vial contains >5 x 10^5 cells in 1 ml volume. HDF-a are characterized by their spindle morphology and immunofluorescent method with antibody to fibronectin. HDF-a are negative for HIV-1, HBV, HCV, mycoplasma, bacteria, yeast and fungi. HDF-a are guaranteed to further expand for 15 population doublings at the condition provided by Creative Bioarray.
Never can primary cells be kept at -20 °C.
Human dermal fibroblasts-adult (HDF-a) is isolated from adult skin and is a key cell that constitutes the loose connective tissue of the skin. Typically displaying a spindle-shaped or stellate flattened structure, they possess prominent cellular protrusions and a large, distinct nucleus. There are two subsets of these cells: papillary fibroblasts and reticular fibroblasts. Papillary fibroblasts adjacent to the epidermis, located in the upper dermis, are proliferative, participate actively in immune activation, and help preserve epidermal shape. Reticular fibroblasts, on the other hand, sit in the deep dermis, protected by an extracellular matrix layer, and are best used to support cytoskeletal remodeling and migration.
Dermal fibroblasts are important to skin morphology and are easily cultured in vitro, making them ubiquitously used for cellular and molecular studies. With HDFs combined with organic scaffolds or biocompatible extracellular matrices, it is possible to build sophisticated artificial tissues, organs and even human counterparts. HDFs are also used in the manufacture of skin repair products, such as synthetic dermis and skin grafts. The less effective fibroblasts are with age, the more skin can become wrinkled and lax. The dysregulation of fibroblasts occurs with conditions, including scleroderma and fibrotic disorders. Thus, HDFs are the perfect models for exploring the pathology and therapeutic potential of these disorders.
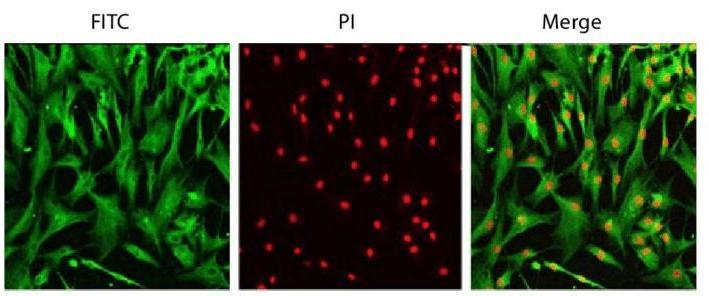 Fig. 1. Hman dermal fibroblast cell line staining with DDR-2 antibody and PI (Yun J, Park H, et al., 2010).
Fig. 1. Hman dermal fibroblast cell line staining with DDR-2 antibody and PI (Yun J, Park H, et al., 2010).
Inhibition Effects of Inotodiol on Oxidative Stress-Induced NOX5, MAPK and NF-ΚB in HDF Cells
Skin aging is a process driven by both internal influences like changes in collagen levels, and external influence like pollution, sunlight and oxidative damage. Inotodiol is a lanostane triterpenoid found in Inonotus obliquus and known to be antiviral, anticancer and anti-inflammatory; however, it is unknown whether it blocks oxidative stress-related aging of human skin.
Lee's team examined the effect of inotodiol on oxidative stress in human dermal fibroblasts (HDF). ROS, specifically H2O2, are major sources of oxidative stress that damage cells and causes ageing. NOX5 is an important mediator of ROS production and is mediated by the MAPK pathway. They tested NOX5 expression in HDFs using WB and found that NOX5 expression increased in response to hydrogen peroxide stimulation in line with the number of NOX5 gene transcripts. HDF cells treated with inotodiol showed decreased hydrogen peroxide-mediated NOX5 production (Fig. 1a and b). WB expression of MAPK and NF-B was then checked and, after hydrogen peroxide stimulation, MAPK and NF-B were increased. Inotodiol-treated HDF cells displayed decreased hydrogen peroxide-induced increases in MAPK and NFB activity (Fig. 2). They used ELISA kits to detect phospho-ERK1/2 in HDF cells to see whether inotodiol regulated oxidative stress-mediated activation of ERK1/2. Phospho-ERK1/2 was elevated after treatment with H2O2; they were decreased after treatment with inotodiol. Inotodiol therefore reduces HDF-cell oxidative stress-dependent ERK1/2 and NFB expression.
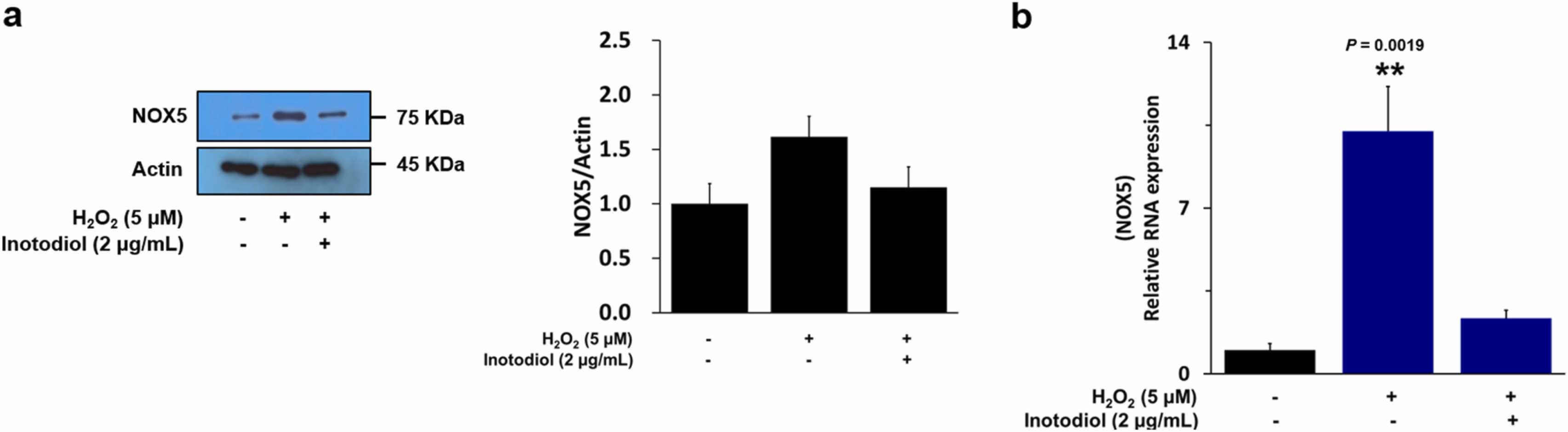 Fig. 1. The effects of inotodiol on oxidative stress-induced NOX5 activation in HDF cells (Lee SH, Won GW, et al., 2022).
Fig. 1. The effects of inotodiol on oxidative stress-induced NOX5 activation in HDF cells (Lee SH, Won GW, et al., 2022).
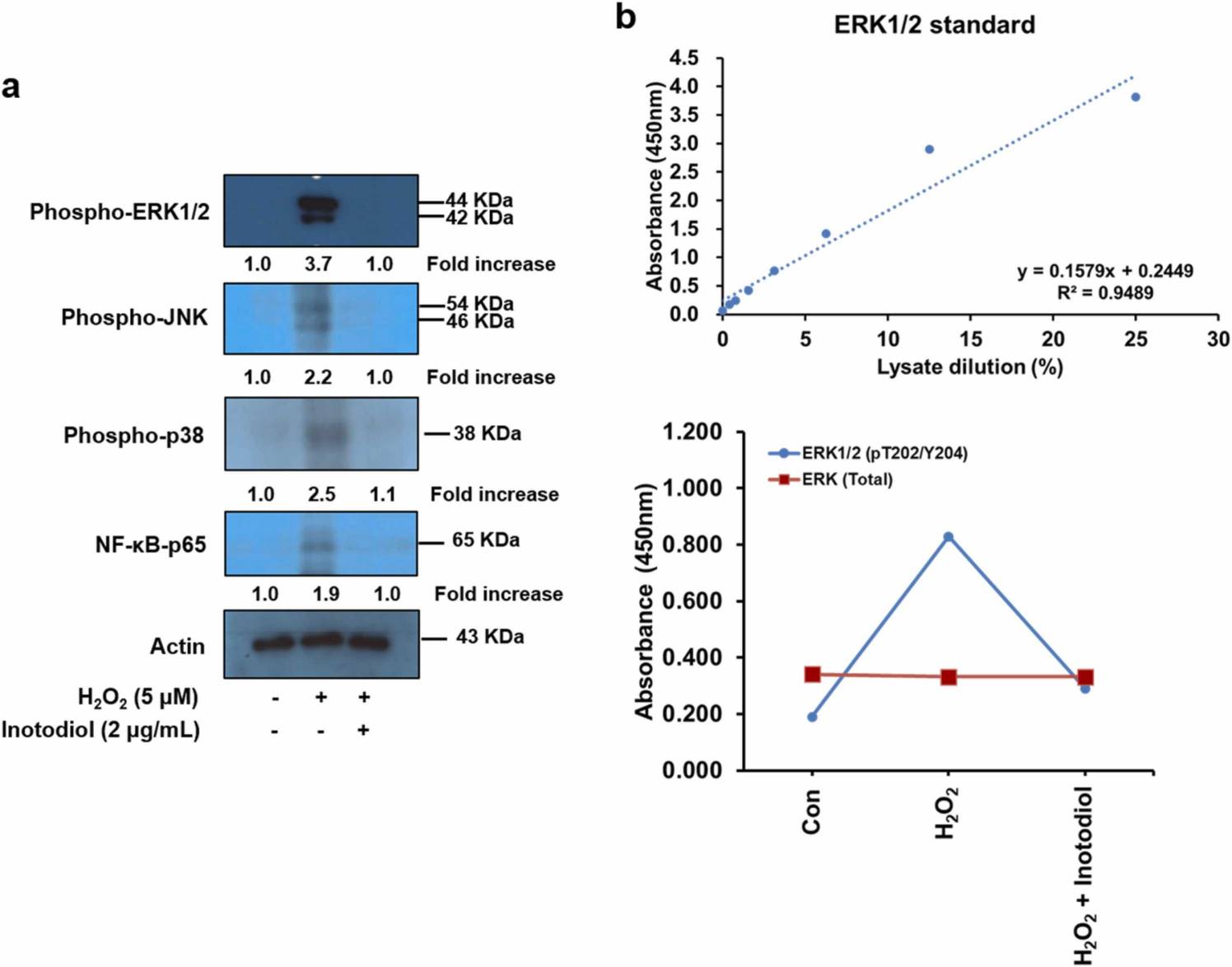 Fig. 2. The effects of inotodiol on oxidative stress-induced p-ERK1/2 and NF-κB-p65 activation in HDF cells (Lee SH, Won GW, et al., 2022).
Fig. 2. The effects of inotodiol on oxidative stress-induced p-ERK1/2 and NF-κB-p65 activation in HDF cells (Lee SH, Won GW, et al., 2022).
Effects of HASC-derived EVs on the Migration of UVB-irradiated HDFs
Aging occurs due to both internal mechanisms (natural ageing) and external factors (UV rays, leading to photoaging with increased ROS production causing collagen and elastin degradation). More recently, EVs have attracted attention for their ability to regenerate skin.
Choi's team tested EVs from human adipose-derived stem cells (HASCs) against UVB-induced photoaging of human dermal fibroblasts (HDFs). Normal HDFs were given serum-free medium and HASC-derived EVs. The EVs boosted type I collagen production to an exponential rate dependent on dose, peaking at 5×108 particles/mL. EVs also induced collagen production in UVB-irradiated HDFs without significant differences between 1 and 5 × 108 particles/mL (Fig. 3). For early cellular absorption, normal and UVB-treated HDFs were treated for 3 hours with 1×108 particles/mL of fluorescent dye-labeled EVs. The EVs were internalized into the cytoplasm in both types of cells within this time, indicating high uptake efficiency. For migration studies, a scratch wound healing and transwell migration assay were performed. UVB-irradiated HDFs generally recovered slower than normal HDFs. However, wounds in UVB-irradiated HDFs treated with EVs closed faster than those without EVs or with BSA treatment (Fig. 4). In the transwell migration assay, normal HDFs migrated to the lower chamber with GM or EVs, while UVB-irradiated HDFs showed lower migration but increased migration with EVs. Also, EVs enhanced the proliferation of UVB-irradiated HDFs after 48 hours (Fig. 5). These findings suggest that HASC-derived EVs improve the migration and proliferation of UVB-irradiated HDFs.
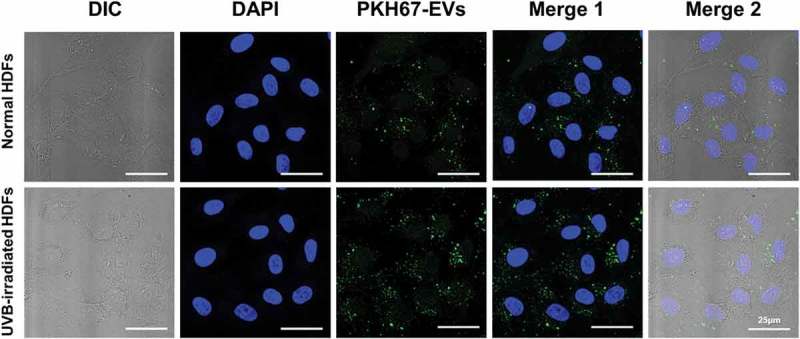 Fig. 3. The cellular uptake of HASC-derived EVs in normal and UVB-irradiated HDFs (Choi JS, Cho WL, et al., 2019).
Fig. 3. The cellular uptake of HASC-derived EVs in normal and UVB-irradiated HDFs (Choi JS, Cho WL, et al., 2019).
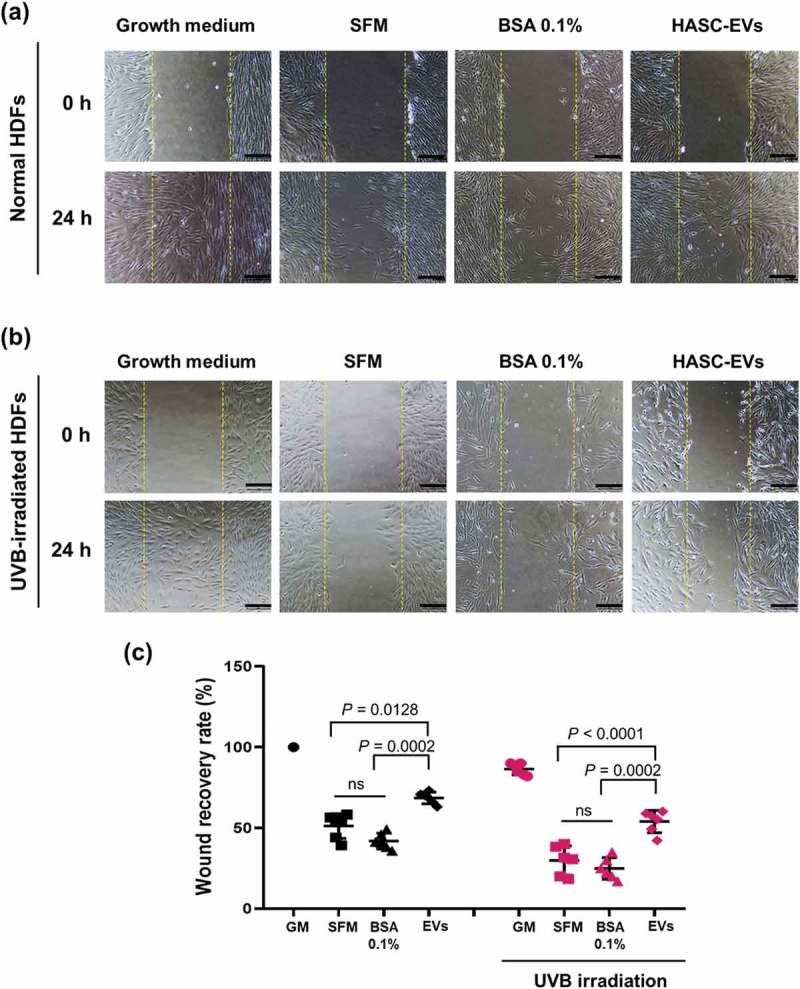 Fig. 4. The effects of HASC-derived EVs on the wound recovery of UVB-irradiated HDFs (Choi JS, Cho WL, et al., 2019).
Fig. 4. The effects of HASC-derived EVs on the wound recovery of UVB-irradiated HDFs (Choi JS, Cho WL, et al., 2019).
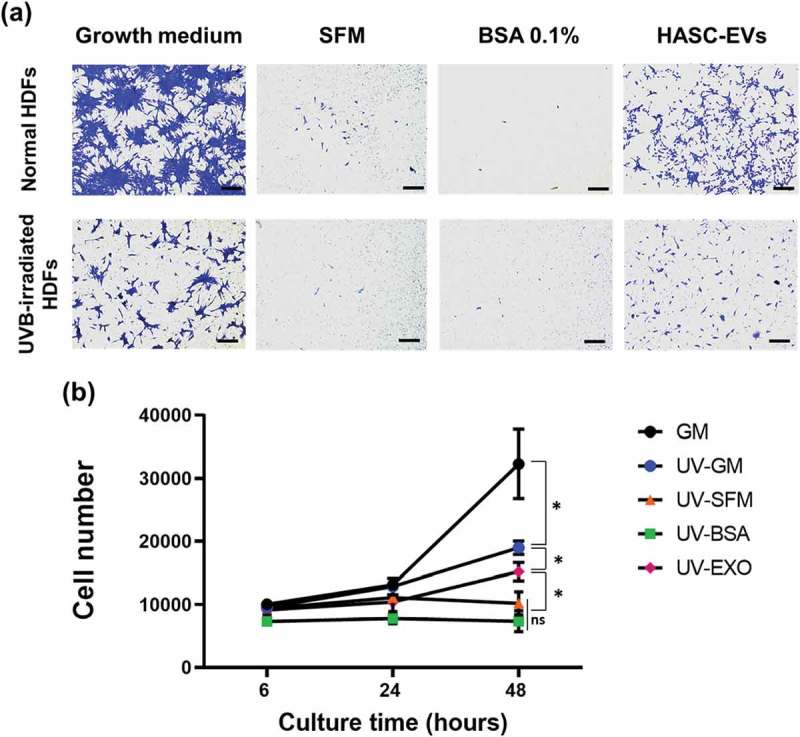 Fig. 5. The effects of HASC-derived EVs on the (a) migration and (b) proliferation of UVB-irradiated HDFs (Choi JS, Cho WL, et al., 2019).
Fig. 5. The effects of HASC-derived EVs on the (a) migration and (b) proliferation of UVB-irradiated HDFs (Choi JS, Cho WL, et al., 2019).
Ask a Question
Write your own review